Nitrosyl chloride
Nitrosyl chloride is the chemical compound with the formula NOCl. It is a yellow gas that is most commonly encountered as a decomposition product of aqua regia, a mixture of hydrochloric acid and nitric acid. It is a strong electrophile and oxidizing agent. It is sometimes called Tilden's reagent.
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Nitrosyl chloride | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.018.430 |
| EC Number |
|
| E number | E919 (glazing agents, ...) |
| MeSH | nitrosyl+chloride |
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 1069 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| NOCl | |
| Molar mass | 65.459 g mol−1 |
| Appearance | Yellow gas |
| Density | 2.872 mg mL−1 |
| Melting point | −59.4 °C (−74.9 °F; 213.8 K) |
| Boiling point | −5.55 °C (22.01 °F; 267.60 K) |
| Reacts | |
| Structure | |
| Dihedral, digonal | |
| Hybridisation | sp2 at N |
| 1.90 D | |
| Thermochemistry | |
Std molar entropy (S |
261.68 J K−1 mol−1 |
Std enthalpy of formation (ΔfH⦵298) |
51.71 kJ mol−1 |
| Hazards | |
| Safety data sheet | inchem.org |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Related compounds |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Structure and synthesis
The molecule is bent. A double bond exists between N and O (distance = 1.16 Å) and a single bond between N and Cl (distance = 1.96 Å). The O–N–Cl angle is 113°.[1]
Production
Since nitrosyl chloride is chemically simple and thermally stable, it can be produced in many ways.
- Combining nitrosylsulfuric acid and HCl affords the compound. This method is used industrially.[2]
- HCl + NOHSO4 → H2SO4 + NOCl
- A more convenient laboratory method involves the (reversible) dehydration of nitrous acid by HCl[3]
- HNO2 + HCl → H2O + NOCl
- Michael Faraday prepared nitrosyl chloride by reacting palladium with aqua regia:
- Pd + HNO3 + 3 HCl → PdCl2 + 2 H2O + NOCl
- NOCl forms by the direct combination of chlorine and nitric oxide; This reaction reverses above 100 °C.
- Cl2 + 2 NO → 2NOCl
- Another method of producing nitrosyl chloride is by direct union of the elements at 400 °C, although there is some regression as above.
- N2 + O2 + Cl2 → 2 NOCl ⇌ 2 NO + Cl2
Occurrence in aqua regia
NOCl also arises from the combination of hydrochloric and nitric acids according to the following reaction:[4]
- HNO3 + 3 HCl → Cl2 + 2 H2O + NOCl
In nitric acid, NOCl is readily oxidized into nitrogen dioxide. The presence of NOCl in aqua regia was described by Edmund Davy in 1831.[5]
Reactions
NOCl behaves as an electrophile and an oxidant in most of its reactions. With halide acceptors, for example antimony pentachloride, converts to nitrosonium salts:
- NOCl + SbCl5 → [NO]+[SbCl6]−
In a related reaction, sulfuric acid gives nitrosylsulfuric acid, the mixed acid anhydride of nitrous and sulfuric acid:
- ClNO + H2SO4 → ONHSO4 + HCl
NOCl reacts with silver thiocyanate to give silver chloride and the pseudohalogen nitrosyl thiocyanate:
- ClNO + AgSCN → AgCl + ONSCN
Similarly, it reacts with silver cyanide to give nitrosyl cyanide.[6]
Nitrosyl chloride is used to prepare metal nitrosyl complexes. With molybdenum hexacarbonyl, NOCl gives the dinitrosyldichloride complex:[7]
- Mo(CO)6 + 2 NOCl → MoCl2(NO)2 + 6 CO
Applications in organic synthesis
Aside from its role in the production of caprolactam, NOCl finds some other uses in organic synthesis. It adds to alkenes to afford α-chloro oximes.[8] The addition of NOCl follows the Markovnikov rule. Ketenes also add NOCl, giving nitrosyl derivatives:
- H2C=C=O + NOCl → ONCH2C(O)Cl
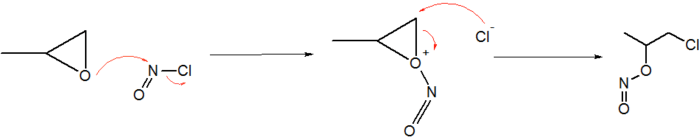
Epoxides react with NOCl to give an α-chloronitritoalkyl derivatives. In the case of propylene oxide, the addition proceeds with high regiochemistry:[9]
It converts amides to N-nitroso derivatives.[10] NOCl converts some cyclic amines to the alkenes. For example, aziridine reacts with NOCl to give ethene, nitrous oxide and hydrogen chloride.
Industrial applications
NOCl and cyclohexane react photochemically to give cyclohexanone oxime hydrochloride. This process exploits the tendency of NOCl to undergo photodissociation into NO and Cl radicals. The oxide is converted to caprolactam, a precursor to Nylon-6.[2]
Safety
Nitrosyl chloride is very toxic and irritating to the lungs, eyes, and skin.
References
- Holleman, A. F.; Wiberg, E. (2001). Inorganic Chemistry. San Diego: Academic Press. ISBN 0-12-352651-5.
- Ritz, Josef; Fuchs, Hugo; Kieczka, Heinz; Moran, William C. (2002). "Caprolactam". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a05_031.
- Morton, J. R.; Wilcox, H. W. (1953). "Nitrosyl Chloride". Inorganic Syntheses. 48: 52. doi:10.1002/9780470132357.ch16.
- Beckham, L. J.; Fessler, W. A.; Kise, M. A. (1951). "Nitrosyl Chloride". Chemical Reviews. 48 (3): 319–396. doi:10.1021/cr60151a001.
- Edmund Davy (1830–1837). "On a New Combination of Chlorine and Nitrous Gas". Abstracts of the Papers Printed in the Philosophical Transactions of the Royal Society of London. 3: 27–29. JSTOR 110250.
- Kirby, G. W. (1977). "Tilden Lecture. Electrophilic C-nitroso-compounds". Chemical Society Reviews. 6: 1. doi:10.1039/CS9770600001.
- Johnson, B. F. G.; Al-Obadi, K. H. (1970). "Dihalogenodinitrosylmolybdenum and Dihalogenodinitrosyltungsten". Inorg. Synth. 12: 264–266. doi:10.1002/9780470132432.ch47.
- Ohno, M.; Naruse, N.; Terasawa, I. (1969). "7-cyanoheptanal". Org. Synth. 49: 27. doi:10.15227/orgsyn.049.0027.
- Malinovskii, M. S.; Medyantseva, N. M. (1953). "Olefin Oxides. IX. Condensation of Olefin Oxides with Nitrosyl Chloride". Zhurnal Obshchei Khimii. 23: 84-6. (translated from Russian)
- Van Leusen, A. M.; Strating, J. (1977). "p-Tolylsulfonyldiazomethane". Org. Synth. 57: 95. doi:10.15227/orgsyn.057.0095.
External links