Nitrosyl cyanide
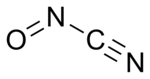
Nitrosyl cyanide is the compound with the molecular formula ONCN. It is a blue-green gas.[1] The compound has been invoked as a product of the oxidation of cyanamide catalyzed by the enzyme glucose oxidase.[2]
 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| CN2O | |
| Molar mass | 56.024 g·mol−1 |
| Appearance | blue-green gas |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Structure, synthesis, reactivity
In terms of its structure, nitrosyl cyanide is planar. It is strongly bent at the internal nitrogen, analogous to the structure of nitrosyl chloride. The C-N-O angle is 113°. The NCN angle is 170°.[1]
The compound can be generated by the reaction of nitrosyl chloride and silver cyanide at low temperatures. It is not typically isolated, but trapped by Diels-Alder reactions, e.g. with butadiene. Cycloadditions occur across the N=O bond. It forms a reversible adduct with 9,10-dimethylantracene.[1]
Related compound
- Nitryl cyanide (O2NCN), a colorless gas (b.p. 7 °C).[3]
gollark: Heresy!
gollark: Oh, I wanted to know whether you thought the EmuWiki™ should use DokuWiki or MediaWiki.
gollark: On https://notes.osmarks.tk/ you can do https://notes.osmarks.tk/bees:potatoes and stuff.
gollark: It's just not been set up here.
gollark: There is a fancy URLs mode in Dokuwiki.
References
- Kirby, G. W. (1977). "Tilden Lecture. Electrophilic C-nitroso-compounds". Chemical Society Reviews. 6: 1. doi:10.1039/CS9770600001.
- Shirota, Frances N.; Goon, David J.W.; Demaster, Eugene G.; Nagasawa, Herbert T. (1996). "Nitrosyl cyanide, a putative metabolic oxidation product of the alcohol-deterrent agent cyanamide". Biochemical Pharmacology. 52 (1): 141–147. doi:10.1016/0006-2952(96)00174-8. PMID 8678898.
- Rahm, Martin; Bélanger-Chabot, Guillaume; Haiges, Ralf; Christe, Karl O. (2014). "Nitryl Cyanide, NCNO2". Angewandte Chemie International Edition. 53 (27): 6893–6897. doi:10.1002/anie.201404209. PMID 24861214.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.