Diisobutylaluminium hydride
Diisobutylaluminium hydride (DIBALH, DIBAL, DIBAL-H or DIBAH, /ˈdaɪbæl/ DY-bal) is a reducing agent with the formula (i-Bu2AlH)2, where i-Bu represents isobutyl (-CH2CH(CH3)2). This organoaluminium compound was investigated originally as a co-catalyst for the polymerization of alkenes.[1]
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Diisobutylaluminum hydride | |
| Other names
DIBAH; DIBAL; DiBAlH; DIBAL-H; DIBALH | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.013.391 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C8H19Al (monomer) C16H38Al2 (dimer) | |
| Molar mass | 142.22 g/mol (monomer) 284.44 g/mol (dimer) |
| Appearance | Colorless liquid |
| Density | 0.798 g/cm3 |
| Melting point | −80 °C (−112 °F; 193 K) |
| Boiling point | 116 to 118 °C (241 to 244 °F; 389 to 391 K) at 1 mmHg |
| Violently reacts with water | |
| Solubility in hydrocarbon solvents | Soluble |
| Hazards | |
| Main hazards | ignites in air |
| GHS pictograms |   |
| GHS Signal word | Danger |
GHS hazard statements |
H220, H225, H250, H260, H314, H318 |
| P210, P222, P223, P231+232, P233, P240, P241, P242, P243, P260, P264, P280, P301+330+331, P302+334, P303+361+353, P304+340, P305+351+338, P310, P321, P335+334, P363, P370+378, P377, P381, P402+404 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Properties
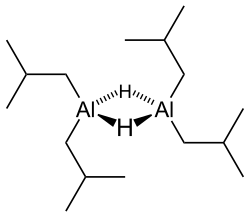
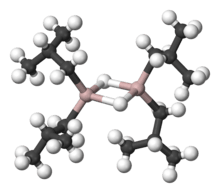
Like most organoaluminum compounds, the compound’s structure is most probably more than that suggested by its empirical formula. A variety of techniques, not including X-ray crystallography, suggest that the compound exists as a dimer and a trimer, consisting of tetrahedral aluminium centers sharing bridging hydride ligands.[2] Hydrides are small and, for aluminium derivatives, are highly basic, thus they bridge in preference to the alkyl groups.
DIBAL can be prepared by heating triisobutylaluminium (itself a dimer) to induce beta-hydride elimination:[3]
- (i-Bu3Al)2 → (i-Bu2AlH)2 + 2 (CH3)2C=CH2
Although DIBAL can be purchased commercially as a colorless liquid, it is more commonly purchased and dispensed as a solution in an organic solvent such as toluene or hexane.
Use in organic synthesis
DIBAL is useful in organic synthesis for a variety of reductions, including converting carboxylic acids, their derivatives, and nitriles to aldehydes. DIBAL efficiently reduces α-β unsaturated esters to the corresponding allylic alcohol.[4] By contrast, LiAlH4 reduces esters and acyl chlorides to primary alcohols, and nitriles to primary amines [use Feiser work-up procedure]. DIBAL reacts slowly with electron-poor compounds, and more quickly with electron-rich compounds. Thus, it is an electrophilic reducing agent whereas LiAlH4 can be thought of as a nucleophilic reducing agent.
Although DIBAL reliably reduces nitriles to aldehydes, the reduction of esters to the same functional group is an infamously finicky reaction which looks useful on paper but often leads to mixtures of alcohol and aldehyde in practice. This problem has been addressed by careful control of the reaction conditions using continuous flow chemistry.[5]
Safety
DIBAL, like most alkylaluminium compounds, reacts violently with air and water, potentially leading to fires.
References
- Ziegler, K.; Martin, H.; Krupp, F. (1960). "Metallorganische Verbindungen, XXVII Aluminiumtrialkyle und Dialkyl-Aluminiumhydride aus Aluminiumisobutyl-Verbindungen". Justus Liebigs Annalen der Chemie. 629 (1): 14–19. doi:10.1002/jlac.19606290103.
- Self, M. F.; Pennington, W. T.; Robinson, G. H. (1990). "Reaction of Diisobutylaluminum Hydride with a Macrocyclic Tetradentate Secondary Amine. Synthesis and Molecular Structure of [Al(iso-Bu)]2[C10H20N4][Al(iso-Bu)3]2: Evidence of an Unusual Disproportionation of (iso-Bu)2AlH". Inorganica Chimica Acta. 175 (2): 151–153. doi:10.1016/S0020-1693(00)84819-7.
- Eisch, J. J. (1981). Organometallic Syntheses. 2. New York: Academic Press. ISBN 0-12-234950-4.
- Galatsis, P. (2001). "Diisobutylaluminum Hydride". Encyclopedia of Reagents for Organic Synthesis. New York: John Wiley & Sons. doi:10.1002/047084289X.rd245. ISBN 0471936235.
- Webb, Damien; Jamison, Timothy F. (2012-01-20). "Diisobutylaluminum Hydride Reductions Revitalized: A Fast, Robust, and Selective Continuous Flow System for Aldehyde Synthesis". Organic Letters. 14 (2): 568–571. doi:10.1021/ol2031872. hdl:1721.1/76286. ISSN 1523-7060. PMID 22206502.
External links
- Stockman, R. (2001). "Dibal reduction of an amino acid derived methyl ester; Garner's Aldehyde". ChemSpider Synthetic Pages. doi:10.1039/SP161. SyntheticPage 161.
- "Oxidation And Reduction Reactions in Organic Chemistry". University of Southern Maine, Department of Chemistry. Archived from the original on 2011-06-11.
- "Diisobutyl Aluminum hydride (DIBAL-H) and Other Isobutyl Aluminum Alkyls (DIBAL-BOT, TIBAL) as Specialty Organic Synthesis Reagents" (PDF). Akzo-Nobel. Archived from the original (PDF) on 2011-04-08. Retrieved 2011-02-23.