Gobiesocidae
Clingfishes are fishes of the family Gobiesocidae, the only family in the order Gobiesociformes. These fairly small to very small fishes are widespread in tropical and temperate regions, mostly near the coast, but a few species in deeper seas or fresh water. Most species shelter in shallow reefs or seagrass beds, clinging to rocks, algae and seagrass leaves with their sucking disc, a structure on their chest.[1][2]
| Clingfishes | |
|---|---|
 | |
| Aspasmichthys ciconiae | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Actinopterygii |
| Clade: | Percomorpha |
| (unranked): | Ovalentaria |
| Order: | Gobiesociformes |
| Family: | Gobiesocidae Bleeker, 1860 |
| Type species | |
| Gobiesox cephalus Lacépède, 1800 | |
They are generally too small to be of interest to fisheries, although the relatively large Sicyases sanguineus regularly is caught as a food fish,[3] and some of the other species occasionally appear in the marine aquarium trade.[1]
Distribution and habitat
.jpg)

Clingfishes are primarily found near the shore in the Atlantic, Indian and Pacific Oceans, including marginal seas such as the Mediterranean, Gulf of Mexico, Caribbean and Gulf of California. The greatest species richness is in tropical and warm temperate regions, but the range of a few extends into colder waters, like Diplecogaster bimaculata (north to Norway), Apletodon dentatus, Lepadogaster candolii and L. purpurea (all three north to Scotland; the last formerly mistaken for the mostly Mediterranean L. lepadogaster), Gobiesox maeandricus (north to Alaska), Gobiesox marmoratus and Sicyases sanguineus (both to southernmost South America), and Gastrocymba quadriradiata (from New Zealand's subantarctic islands).[6][7][8][9][10]
Clingfishes mainly inhabit shallow rocky reefs and shores, coral reefs, seagrass meadows and algae beds. They often live in places exposed to strong currents and wave action, and some are amphibious. As long as the strongly amphibious, intertidal-living species are kept moist by splashing waves, they can survive for up to three–four days on land, gaining oxygen from the air by the branchial surfaces (gills), skin and perhaps the mouth.[4][11][12] At least a few species even tolerate a relatively high degree of water loss when on land.[4]
A relatively small number of species shelter in sea urchins or crinoids. Whether this relationship is obligate (clingfish always with a sea urchin or crinoid) or facultative (clingfish sometimes with a sea urchin or crinoid) varies with species. In some, only young clingfish are obligate and gradually move away as they become adult.[13][14][15] Three clingfish species, the Australian Cochleoceps bicolor and C. orientalis, and the warm East Atlantic Diplecogaster tonstricula, are cleaner fish that will cling onto the bodies of larger fish.[1][16][17]
Although several species can occur in brackish water, only seven (Gobiesox cephalus, G. fluviatilis, G. fulvus, G. juniperoserrai, G. juradoensis, G. mexicanus and G. potamius) from warmer parts of the Americas are freshwater fish that live in fast-flowing rivers and streams.[18][19]
Most known clingfish species are from relatively shallow coastal waters, but several inhabit the mesophotic zone and a few even deeper, with Alabes bathys, Gobiesox lanceolatus, Gymnoscyphus ascitus, Kopua kuiteri, K. nuimata and Protogobiesox asymmetricus reported from depths of 300–560 m (980–1,840 ft).[20][21] Because of their small size and typical habitat, it is however suspected that still-undiscovered deep-water species remain.[20] Even in shallow coastal waters many clingfish are highly cryptic and easily overlooked, mostly staying under cover, although there are species that are active and will swim in the open.[22] As a consequence their abundance is often not well known. Several species are only known from a single or a few specimens.[20][21][23] Species that appear uncommon or rare based on standard methods can actually be common if using methods that are more suitable for detecting them.[24] Studies of better-known species have shown that they can be locally abundant. As many as 23 individuals of Lepadogaster lepadogaster have been documented from a single square metre (more than two individuals per square foot).[25] As of 2018, the IUCN has evaluated the conservation status of 84 clingfish species (roughly half the species in the family). The majority of these are considered least concern (not threatened), 17 are considered data deficient (available data prevents an evaluation), 8 considered vulnerable and a single endangered. The vulnerable and endangered species all have small distributions, restricted to islands or a single bay.[26] Three Gobiesox species that are restricted to fresh water in Mexico have not been rated by the IUCN, but are considered threatened by Mexican authorities.[27]
Description
Clingfishes are typically small fish, with most species less than 7 cm (2.8 in) in length,[28] and the smallest no more than 1.5 cm (0.6 in).[1] Only a few species can surpass 12 cm (4.7 in) in length and the largest, Chorisochismus dentex and Sicyases sanguineus, both reach up to 30 cm (12 in).[4][29] Males typically grow larger than females.[2]
Most clingfish species have tapering bodies and flattened heads, appearing somewhat tadpole-like in their overall shape. They lack a swim bladder. The lateral line of clingfish is well developed, but may not extend to the posterior parts of the body. The skin of clingfishes is smooth and scaleless, with a thick layer of protective mucus.[2] In at least Diademichthys lineatus and Lepadichthys frenatus, the mucus production increases if the fish is disturbed. The taste of their mucus is highly bitter to humans and it can kill other fish. This is due to their skin and mucus containing a grammistin-like toxin (the toxin in soapfish, such as Grammistes). Whether any other clingfish has toxins in its skin or mucus is currently unknown.[5][30] Another defense appears to be present in a couple of Acyrtus and Arcos species. They have a spine at their gill cover and it appears to be connected to a venom gland. Although the evidence presently is circumstantial, this strongly suggests that the world's smallest venomous fish is Acyrtus artius, which is less than 3 cm (1.2 in) long.[31][32]
Sucking disc
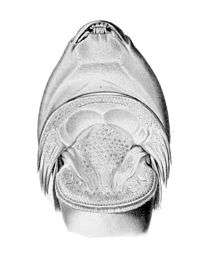
Clingfish are named for their ability to firmly attach themselves to various surfaces, even in strong water currents or when battered by waves. This ability is aided by their sucking disc, which is located on the underside at the chest and is formed primarily by modified pelvic fins and adjacent tissue.[2][4][12][28] In some species it is divided in two, resulting in a larger front and a smaller rear sucking disc.[2] The sucking disc is covered in tiny hexagons and each of these consists of many microscopic hair-like structures (setae). This is similar to the structures that allow geckos to cling to walls. The sucking disc can be remarkably strong, in some species able to lift as much as 300 times the weight of the clingfish.[12] Gobies (family Gobiidae) can have a similar sucking disc, but unlike that family the single dorsal fin in clingfish is not spiny.[2] In a few clingfish species the disc is reduced or even absent, notably Alabes, which are quite eel-like in their shape and aptly named shore-eels.[1][16] The sucking disc is also reduced in some deep-water clingfish species.[12]
Colours



.jpg)
- Gobiesox rhessodon (top left) is a typical, cryptic clingfish.
- Lepadogaster purpurea (top right) has two eyespots on top of its head.
- Diademichthys lineatus (bottom left) has a striped disruptive pattern that may function as camouflage when between sea urchin spines, but may also be warning colours as it is poisonous
- Cochleoceps orientalis (bottom right) is a quite brightly coloured cleaner fish.
Most clingfish species have a cryptic colouration, often brown, grey, whitish, black, reddish or green shades, and in some cases they can rapidly change colour to match their background.[2][33][34]
Species of deep water are often orange-red (these long wave-length colours are the first that disappear with depth, making them suitable for camouflage).[21] Diademichthys lineatus, Discotrema species, Lepadichthys caritus and L. lineatus are strongly banded, which may function as a disruptive pattern when among sea urchin spines or crinoid arms, but may also be warning colours, as some members of these genera have poisonous skin and mucus (it is unknown if all of them are poisonous).[5][13][30] There are species with colours or patterns that are unsuitable for camouflage. Although Lepadogaster purpurea overall is cryptic, it has a pair of distinct large eyespots on the top of its head.[9] Cochleoceps bicolor, C. orientalis and Diplecogaster tonstricula are yellow to red with fine bluish lines. These three are cleaner fish.[16][17]
Feeding
Feeding varies depending on exact clingfish species. Most primarily feed on tiny crustaceans (such as amphipods, copepods, isopods, mysids, ostracods and shrimp) or gastropods (limpets and other sea snails). Other small animals that have been recorded in their diet include chitons, bivalves, medium-small crustacean like crabs and barnacles, sea urchins, worms, insect larvae, fish and fish eggs.[2][3][34][35] In some species, cannibalism where a large clingfish eats a smaller clingfish is not uncommon.[34][36]
Limpets and other shelled invertebrates are well-protected and often strongly attached to the rock surface. Clingfish species that feed extensively on them have developed specialized teeth and techniques to dislodge them. This includes rapidly inserting their relatively large, fang-like front teeth under the edge of the prey to flip it, or jamming the teeth on or under the shell's edge to make a small break.[11][12][29] However, the teeth of clingfish vary extensively depending on species.[37][38] In the opposite extreme of the species with relatively few large teeth is Nettorhamphos radula. This species has 1,800–2,300 microscopic teeth (about ten times more than known from any other clingfish), but its feeding behavior is unknown.[23][37]
Three clingfish species, Cochleoceps bicolor, C. orientalis and Diplecogaster tonstricula, have become cleaner fish. Large fish approach them and allow the small clingfish onto their body where the clingfish eats tiny parasites.[16][17] In contrast to this mutualistic relationship, certain clingfish species that live among the spines of sea urchins appear to be part of a more varied relationship. It can be either commensal (the clingfish gains protection from the sea urchin spines, but apparently neither benefits nor is a disadvantage to the sea urchin) or parasitic (the clingfish gains protection, and eats tube feet and pedicellaria from its sea urchin host).[14][15][39]
No clingfish species is known to be exclusively herbivorous, but some are omnivorous and will feed extensively on a range of algae (brown, green and red),[3] while other, more strictly carnivorous species may ingest plant material incidentally.[34]
Classification and taxonomy
The classification of the clingfishes varies. FishBase places Gobiesocidae as the only family in the order Gobiesociformes, under the superorder Paracanthopterygii;[40] whereas ITIS place them in the suborder Gobiesocoidei of the order Perciformes, under superorder Acanthopterygii. ITIS lists Gobiesociformes as invalid.[41] The 5th edition of Fishes of the World places the Gobiesociiformes in the clade Percomorpha as part of the series Ovalentaria.[42]
Mostly being very small and often cryptic, new species are regularly discovered and described. A major authoritative work on the family is a monograph that was published in 1955 by J.C. Briggs,[43] but in the half century after its publication, up until 2006, fifty-six new clingfish species were described, or on average more than one per year.[5] This pattern with regular descriptions of new species—and even new genera—has continued since then.[37][44][45][46] As of 2018, there are more than 170 recognized clingfish species.[46]
Subfamilies and genera

Subfamilies and genera. The delimination of the subfamilies, and to some extent the genera, is not fully resolved.[22][46] The 5th edition of Fishes of the World recognises only two subfamilies, Cheilobranchinae and Gobiesocinae.[42] Fishbase does list a third subfamily, the monotypic Protogobiesocinae which contains a single species Protogobiesox asymmetricus, this species having been described in 2016.[47]
Subfamily Cheilobranchinae
Subfamily Gobiesocinae
- Acyrtops
- Acyrtus
- Apletodon
- Arcos
- Aspasma
- Aspasmichthys
- Aspasmodes
- Aspasmogaster
- Barryichthys[48]
- Briggsia
- Chorisochismus
- Cochleoceps
- Conidens
- Creocele
- Dellichthys
- Derilissus
- Diademichthys
- Diplecogaster
- Diplocrepis
- Discotrema
- Eckloniaichthys
- Flexor[46]
- Gastrocyathus
- Gastrocymba
- Gastroscyphus
- Gobiesox
- Gouania
- Gymnoscyphus
- Haplocylix
- Kopua
- Lecanogaster
- Lepadichthys
- Lepadicyathus
- Lepadogaster
- Liobranchia
- Lissonanchus
- Modicus
- Nettorhamphos[37]
- Opeatogenys
- Parvicrepis
- Pherallodichthys
- Pherallodiscus
- Pherallodus
- Posidonichthys
- Propherallodus
- Protogobiesox[45]
- Rimicola
- Sicyases
- Tomicodon
- Trachelochismus
- Unguitrema[44]
References
- Bray, Dianne. "Family GOBIESOCIDAE". Fishes of Australia. Retrieved 29 September 2014.
- Donaldson, T.J. (2004). "Gobiesocoidei (Clingfishes And Singleslits)". encyclopedia.com. Grzimek's Animal Life Encyclopedia. Retrieved 12 October 2018.
- Paine, R.T.; A.R. Palmer (1978). "Sicyases sanguineus: a Unique Trophic Generalist from the Chilean Intertidal Zone". Copeia. 1978 (1): 75–81. doi:10.2307/1443824. JSTOR 1443824.
- Graham, J.B., ed. (1997). Air-Breathing Fishes: Evolution, Diversity, and Adaptation. Academic Press. pp. 41–42. ISBN 978-0-12-294860-2.
- Craig, M.T.; J.E. Randall (2008). "Two New Species of the Indo-Pacific Clingfish Genus Discotrema (Gobiesocidae)". Copeia. 2008 (1): 68–74. doi:10.1643/CI-07-025.
- Froese, Rainer and Pauly, Daniel, eds. (2018). "Diplecogaster bimaculata" in FishBase. October 2018 version.
- Froese, Rainer and Pauly, Daniel, eds. (2018). "Gobiesox maeandricus" in FishBase. October 2018 version.
- Froese, Rainer and Pauly, Daniel, eds. (2018). "Gastrocymba quadriradiata" in FishBase. October 2018 version.
- Henriques, M.; R. Lourenco; F. Almada; G. Calado; D. Gonçalves; T. Guillemaud; M.L. Cancela; V.C. Almada (2002). "A revision of the status of Lepadogaster lepadogaster (Teleostei: Gobiesocidae): sympatric subspecies or a long misunderstood blend of species?". Biological Journal of the Linnean Society. 76 (1): 327–338. doi:10.1046/j.1095-8312.2002.00067.x.
- Sielfeld, W.; M. Vargas (1999). "Review of marine fish zoogeography of Chilean Patagonia (42°-57°S)". Scientia Marina. 63: 451–463. doi:10.3989/scimar.1999.63s1451.
- Ebeling, A.W.; P. Bernal; A. Zuleta (1970). "Emersion of the amphibious Chiliean clingfish, Sicyastes sanguineus". Biol. Bull. 139 (1): 115–137. doi:10.2307/1540131. JSTOR 1540131. PMID 29332484.
- Simon, M. (6 June 2014). "Absurd Creature of the Week: This Fish Can Support 300 Times Its Weight With a Super Suction Cup". wired.com. Retrieved 12 October 2018.
- Karplus, I. (2014). Symbiosis in Fishes: The Biology of Interspecific Partnerships. Wiley Blackwell. ISBN 9781405185899.
- Sakashita, H. (1992). "Sexual dimorphism and food habits of the clingfish, Diademichthys lineatus, and its dependence on host sea urchin". Environmental Biology of Fishes. 34 (1): 95–101. doi:10.1007/BF00004787.
- Conway, K.W.; A.L. Stewart; A.P. Summers (2018). "A new species of sea urchin associating clingfish of the genus Dellichthys from New Zealand (Teleostei, Gobiesocidae)". ZooKeys (740): 77–95. doi:10.3897/zookeys.740.22712. PMC 5904551. PMID 29674890.
- Hutchins, B.; R. Swainston (1986). Sea Fishes of Southern Australia. Swainston Publishing, Perth. pp. 32–33. ISBN 978-1-86252--661-7.
- Fricke, R.; P. Wirtz; A. Brito (2015). "Diplecogaster tonstricula, a new species of cleaning clingfish (Teleostei: Gobiesocidae) from the Canary Islands and Senegal, eastern Atlantic Ocean, with a review of the Diplecogaster-ctenocrypta species-group". Journal of Natural History. 50 (11–12): 731–748. doi:10.1080/00222933.2015.1079659.
- Conway, K.W.; D. Kim; L. Rüber; H.S. Espinosa Pérez; P.A. Hastings (2017). "Molecular systematics of the New World clingfish genus Gobiesox (Teleostei: Gobiesocidae) and the origin of a freshwater clade". Molecular Phylogenetics and Evolution. 112: 138–147. doi:10.1016/j.ympev.2017.04.024. PMID 28461202.
- Mercado-Silva, N.; J.J. Schmitter-Soto; H. Espinosa-Pérez (2016). "Overlap of mountain clingfish (Gobiesox fluviatilis) and Mexican clingfish (Gobiesox mexicanus) in the Cuitzmala River, Jalisco, Mexico". The Southwestern Naturalist. 61 (1): 83–87. doi:10.1894/0038-4909-61.1.83.
- Hastings, P.A.; K.W. Conway (2017). "Gobiesox lanceolatus, a new species of clingfish (Teleostei: Gobiesocidae) from Los Frailes submarine canyon, Gulf of California, Mexico". Zootaxa. 4221 (3): 393–400. doi:10.11646/zootaxa.4221.3.8. PMID 28187671.
- Moore, G.I.; J.B. Hutchins; M. Okamoto (2012). "A new species of the deepwater clingfish genus Kopua (Gobiesociformes: Gobiesocidae) from the East China Sea—an example of antitropicality?". Zootaxa. 3380: 34–38. doi:10.11646/zootaxa.3380.1.2.
- Almada, F.; M. Henriques; A. Levy; A. Pereira; J. Robalo; V.C. Almada (2008). "Reclassification of Lepadogaster candollei based on molecular and meristic evidence with a redefinition of the genus Lepadogaster". Molecular Phylogenetics and Evolution. 46 (3): 1151–1156. doi:10.1016/j.ympev.2007.05.021. hdl:10400.12/1471. PMID 18280755.
- Ma, M. (17 April 2017). "New many-toothed clingfish discovered with help of digital scans". University of Washington. Retrieved 11 October 2018.
- Craig, M.T.; Williams, J.T. (2015). "Arcos nudus". IUCN Red List of Threatened Species. 2015: e.T185939A1792278. doi:10.2305/IUCN.UK.2015-2.RLTS.T185939A1792278.en.
- Wagner, M.; S. Bračun; M. Kovačić; S.P. Iglésias; D.Y. Sellos; S. Zogaris; S. Koblmüller (2017). "Lepadogaster purpurea (Actinopterygii: Gobiesociformes: Gobiesocidae) from the eastern Mediterranean Sea: Significantly extended distribution range". Acta Ichthyologica et Piscatoria. 47 (4): 417–421. doi:10.3750/AIEP/02244.
- The IUCN Red List of Threatened Species (12 October 2018). "Gobiesocidae". Retrieved 12 October 2018.
- Ceballos, G.; E.D. Pardo; L.M Estévez; H.E. Pérez, eds. (2016). Los peces dulceacuícolas de México en peligro de extinción. pp. 420–426. ISBN 978-607-16-4087-1.
- Froese, Rainer, and Daniel Pauly, eds. (2012). "Gobiesocidae" in FishBase. October 2012 version.
- Stobbs, R.E. (1980). "Feeding Habits of the Giant Clingfish Chorisochismus dentex (Pisces: Gobiesocidae)". South African Journal of Zoology. 15 (3): 146–149. doi:10.1080/02541858.1980.11447702.
- Hori, K.; N. Fusetani; K. Hashimoto; K. Aida; J.E. Randall (1979). "Occurrence of a grammistin-like mucous toxin in the clingfish Diademichthys lineatus". Toxicon. 17 (4): 418–424. doi:10.1016/0041-0101(79)90271-X. PMID 494325.
- Conway, K.W.; C. Baldwin; M.D. White (2014). "Cryptic Diversity and Venom Glands in Western Atlantic Clingfishes of the Genus Acyrtus (Teleostei: Gobiesocidae)". PLOS One. 9 (5): e97664. doi:10.1371/journal.pone.0097664. PMC 4019652. PMID 24825326.
- Keartes, S. (22 May 2014). "Could This be the World's Smallest Venomous Fish?". Earth Touch News. Retrieved 12 October 2018.
- Briggs, J.C.; Hutchins, J.B. (1998). Paxton, J.R.; Eschmeyer, W.N. (eds.). Encyclopedia of Fishes. San Diego: Academic Press. pp. 142–143. ISBN 978-0-12-547665-2.
- Pires, T.H.S.; F.Z. Gibran (2011). "Intertidal life: field observations on the clingfish Gobiesox barbatulus in southeastern Brazil". Neotrop. Ichthyol. 9 (1): 233–240. doi:10.1590/S1679-62252011005000001.
- Hirayama, S.; T. Shiiba; Y. Sakai; H. Hashimoto; K. Gushima (2005). "Fish-egg predation by the small clingfish Pherallodichthys meshimaensis (Gobiesocidae) on the shallow reefs of Kuchierabu-jima Island, southern Japan". Environmental Biology of Fishes. 73 (3): 237–242. doi:10.1007/s10641-005-2260-2.
- Johnson, C.R. (1970). "Notes on the Intertidal Life History of the Northern Clingfish, Gobiesox maeandricus (Girard)". The American Midland Naturalist. 83 (2): 625–627. doi:10.2307/2423966. JSTOR 2423966.
- Conway, K.W.; G.I. Moore; A.P. Summers (2017). "A New Genus and Species of Clingfish (Teleostei: Gobiesocidae) from Western Australia". Copeia. 105 (1): 128–140. doi:10.1643/CI-16-560.
- Conway, K.W.; N.G. Bertrand; Z. Browning; Z.W. Lancon; F.J. Clubb, Jr. (2015). "Heterodonty in the New World: An SEM Investigation of Oral Jaw Dentition in the Clingfishes of the Subfamily Gobiesocinae (Teleostei: Gobiesocidae)". Copeia. 103 (4): 973–998. doi:10.1643/OT-15-234.
- Russell, B.C. (1983). "The food and feeding habits of rocky reef fish of north-eastern New Zealand". New Zealand Journal of Marine and Freshwater Research. 17 (2): 121–145. doi:10.1080/00288330.1983.9515991.
- Froese, Rainer, and Daniel Pauly, eds. (2012). "Gobiesociformes" in FishBase. october 2012 version.
- "Gobiesociformes". Integrated Taxonomic Information System. Retrieved 5 February 2008.
- J. S. Nelson; T. C. Grande; M. V. H. Wilson (2016). Fishes of the World (5th ed.). Wiley. p. 351. ISBN 978-1-118-34233-6.
- Briggs, J.C. (1955). "A monograph of the clingfishes (Order Xenopterygii)". Stanford Ichthyological Bulletin. 6: 1–224.
- Fricke, R. (2014). "Unguitrema nigrum, a new genus and species of clingfish (Teleostei: Gobiesocidae) from Madang, Papua New Guinea" (PDF). Journal of the Ocean Science Foundation. 13: 35–42.
- Fricke R., Chen J.-N., Chen W.-J. (2016). "New case of lateral asymmetry in fishes: A new subfamily, genus and species of deep water clingfishes from Papua New Guinea, western Pacific Ocean". Comptes Rendus Biologies. 340 (1): 47–62. doi:10.1016/j.crvi.2016.11.002.CS1 maint: multiple names: authors list (link)
- Conway, K.W.; A.L. Stewart; A.P. Summers (2018). "A new genus and species of clingfish from the Rangitāhua Kermadec Islands of New Zealand (Teleostei, Gobiesocidae)". ZooKeys (786): 75–104. doi:10.3897/zookeys.786.28539. PMC 6168618. PMID 30283237.
- Froese, Rainer and Pauly, Daniel, eds. (2019). Species of Protogobiesox in FishBase. April 2019 version.
- Conway, K.W.; G.I. Moore; A.P. Summers (2019). "A new genus and two new species of miniature clingfishes from temperate southern Australia (Teleostei, Gobiesocidae)". ZooKeys (864): 35–65. doi:10.3897/zookeys.864.34521. PMC 6646653. PMID 31346309.
External links
- Smith, J.L.B. 1964. The clingfishes of the Western Indian Ocean and the Red Sea. Ichthyological Bulletin; No. 30. Department of Ichthyology, Rhodes University, Grahamstown, South Africa.
| Wikimedia Commons has media related to Gobiesocidae. |
