Cervical screening
Cervical screening[1] is the process of detecting and removing abnormal tissue or cells in the cervix before cervical cancer develops.[2] By aiming to detect and treat cervical neoplasia early on, cervical screening aims at secondary prevention of cervical cancer.[3] Several screening methods for cervical cancer are the Pap test (also known as Pap smear or conventional cytology), liquid-based cytology, the HPV DNA testing and the visual inspection with acetic acid. Pap test and liquid-based cytology have been effective in diminishing incidence and mortality rates of cervical cancer in developed countries but not in developing countries.[4] Prospective screening methods that can be used in low-resource areas in the developing countries are the HPV DNA testing and the visual inspection.[5]
| Cervical screening | |
|---|---|
 Cervical screening testing vehicle in Taiwan, 2012 |
Recommendations
Different countries have different cervical screening recommendations.
In Europe, most countries suggest or offer screening between the ages of 25 to 64.[6] According to the 2010 European guidelines for cervical cancer screening, the age at which to commence screening ranges between 20–30 years of age, "but preferentially not before age 25 or 30 years", depending on burden of the disease in the population and the available resources.[7] In England, the NHS cervical screening programme is available to women aged 25 to 64; women aged 25 to 49 receive an invitation every 3 years and women aged 50 to 64 receive an invitation every 5 years.[8]
In the United States, screening is recommended for women between ages 21–65, regardless of age at sexual initiation or other high-risk behaviors.[9][10][11] For healthy women aged 21–29 who have never had an abnormal Pap smear, cervical cancer screening with cervical cytology (Pap smear) should occur every 3 years, regardless of HPV vaccination status.[12] The preferred screening for women aged 30–65 is "co-testing", which includes a combination of cervical cytology screening and HPV testing, every 5 years.[12] However, it is acceptable to screen this age group with a Pap smear alone every 3 years.[12] In women over the age of 65, screening for cervical cancer may be discontinued in the absence of abnormal screening results within the prior 10 years and no history of high-grade lesions.[12]
In Australia, screening is offered to women aged 18–70, every two years. This is by Pap smear, and regardless of sexual history.[13] In Canada, where screening programmes are arranged at provincial level, the general recommendation is not to begin routine screening until the age of 25 in the absence of specific reasons to, then to screen every three years until the age of 69.[14] But, for example, in Ontario "The Ontario Cervical Screening Program recommends that women who are or have been sexually active have a Pap test every 3 years starting at age 21."[15]
In low-resource countries, decisions regarding cervical screening are made based upon available resources and thus it is often not possible to offer cervical screening as frequently. The greatest impact on cervical cancer reduction appears to result from screening women aged 30 to 39 years, so resources may be directed to that age group.[16]
Types of screening
There are a number of different types of screening method available. In the United States, cervical screening is usually performed using the Pap test (or 'smear test'),[17] though the UK screening programmes changed the screening method to liquid-based cytology in 2008.[18]
Conventional cytology
In the conventional Pap smear, the physician collecting the cells smears them on a microscope slide and applies a fixative. In general, the slide is sent to a laboratory for evaluation.
Studies of the accuracy of conventional cytology report:[19]
- sensitivity 50%
- specificity 94%
Liquid-based monolayer cytology
Since the mid-1990s, techniques based on placing the sample into a vial containing a liquid medium that preserves the cells have been increasingly used. Two of the types are Sure-Path (TriPath Imaging) and Thin-Prep (Cytyc Corp). The media are primarily ethanol-based for Sure-Path and methanol for ThinPrep. Once placed into the vial, the sample is processed at the laboratory into a cell thin-layer, stained, and examined by light microscopy. The liquid sample has the advantage of being suitable for high-risk HPV testing and may reduce unsatisfactory specimens from 4.1% to 2.6%.[20] Proper sample acquisition is crucial to the accuracy of the test, as a cell that is not in the sample cannot be evaluated.
Studies of the accuracy of liquid based monolayer cytology report:
Human papillomavirus testing
Human papillomavirus (HPV) infection is a cause of nearly all cases of cervical cancer.[22] Most women will successfully clear HPV infections within 18 months. Those that have a prolonged infection with a high-risk type[23] (e.g. types 16, 18, 31, 45) are more likely to develop Cervical Intraepithelial Neoplasia, due to the effects that HPV has on DNA.
In 1995, British researchers Anne Szarewski and Jack Cuzick showed that testing for the presence of HPV DNA in cells taken during cervical screening would pick up cases of pre-cancer that were missed by the routine test.[24]
The English National Health Service now includes "HPV triage" in its screening programme. This means that if initial screening test shows borderline results or low-grade abnormal cells, a further test for HPV is made on the sample. If this shows HPV is present, the patient is called for a further examination, but if no HPV is present the patient resumes the usual screening schedule as if no abnormalities had been found.[25]
Studies of the accuracy of HPV testing report:
- sensitivity 88% to 91% (for detecting CIN 3 or higher)[21] to 97% (for detecting CIN2+)[26]
- specificity 73% to 79% (for detecting CIN 3 or higher)[21] to 93% (for detecting CIN2+)[26]
By adding the more sensitive HPV test, the specificity may decline.[27] If the specificity does decline, the result is increased numbers of false positive tests and, for many women that did not have disease, an increased risk for colposcopy, an invasive procedure[28] and unnecessary treatment. A worthwhile screening test requires a balance between the sensitivity and specificity to ensure that those having a disease are correctly identified as having it and those without the disease are not identified as having it.
Regarding the role of HPV testing, randomized controlled trials have compared HPV to colposcopy. HPV testing appears as sensitive as immediate colposcopy while reducing the number of colposcopies needed.[29] randomized controlled trial have suggested that HPV testing could follow abnormal cytology[21] or could precede cervical cytology examination.[26]
A study published in 2007 suggested that the act of performing a Pap smear produces an inflammatory cytokine response, which may initiate immunologic clearance of HPV, therefore reducing the risk of cervical cancer. Women that had even a single Pap smear in their history had a lower incidence of cancer. "A statistically significant decline in the HPV positivity rate correlated with the lifetime number of Pap smears received."[30]
HPV testing can reduce the incidence of grade 2 or 3 Cervical Intraepithelial Neoplasia or cervical cancer detected by subsequent screening tests among women 32–38 years old according to a randomized controlled trial.[31] The relative risk reduction was 41.3%. For patients at similar risk to those in this study (63.0% had CIN 2-3 or cancer), this leads to an absolute risk reduction of 26%. 3.8 patients must be treated for one to benefit (number needed to treat = 3.8). Click here to adjust these results for patients at higher or lower risk of CIN 2-3. One promising prospect in HPV testing is possibility to self-sampling. HPV testing on a self-sample can today be suggested as an additional strategy to reach women not participating in the regular screening programme and in future as a possible screening strategy.[32]
| HPV test | Pap test | Management |
|---|---|---|
| Negative | Negative | Repeat testing in 5 years |
| Any | Negative | Repeat testing in 3 years |
| Negative | Atypical squamous cells of undetermined significance (ASC-US) | Repeat testing in 3 years |
| Negative | Low grade squamous intraepithelial lesion (LSIL) | Repeat testing in 6–12 months |
| Not performed | Atypical squamous cells of undetermined significance (ASC-US) | Repeat testing in 6–12 months |
| Positive | Negative | Repeat testing in 6–12 months |
| Not performed | Low grade squamous intraepithelial lesion (LSIL) | Immediate colposcopy |
| Positive | Low grade squamous intraepithelial lesion (LSIL) | Immediate colposcopy |
| Any | Atypical squamous cells – cannot rule out high grade lesion (ASH-H) | Immediate colposcopy |
| Positive | Atypical squamous cells of undetermined significance (ASC-US) | Immediate colposcopy |
| Any | High-grade squamous intraepithelial lesion (HSIL) | Immediate colposcopy |
| Any | Squamous cell carcinoma (SCC) | Immediate colposcopy |
| Any | Atypical glandular cells (AGC) | Immediate colposcopy |
Screening process
The procedures for testing women using Pap smear, liquid-based cytology, or HPV testing are similar. A sample of cells is collected from the cervix using a spatula or small brush. The cells are then checked for any abnormalities.
To take the sample of cells, the health care clinician inserts an instrument, called a speculum, inside the vagina. The speculum has two arms that spread the walls of the vagina apart in order to see the cervix. Then, they scrape the surface of the cervix with a spatula or small brush. This collects a sample of cells from the outer layer of the cervix.
With a Pap smear, cells collected using a spatula are smeared onto a slide for examination under a microscope. In liquid-based cytology, a sample of cells is taken using a small brush. The cells are put it into a container of liquid, and analysed for abnormalities. Cervical cells to be tested for HPV are collected in a similar way.
Removal of abnormal cells
Women may be told that they have CIN (cervical intraepithelial neoplasia), or CIS (carcinoma in situ) — these terms describe different levels of abnormality found in the cervical cells. Abnormal cells can be removed or destroyed using one of several different procedures.
Laser ablation and cryotherapy treat just the part of the cervix that contains abnormal cells. Laser ablation uses a laser to burn away the abnormal cells, while cryotherapy uses a cold probe to freeze the cells away. These procedures allow normal cells to grow back in their place. The loop electrical excision procedure (called LLETZ or ‘large loop excision of the transformation zone’ in the UK), cervical conization (or cone biopsy) and hysterectomy remove the whole area containing the cells that could become pre-cancerous or develop into cervical cancer.
Testing in resource-poor areas
Many resource-poor areas cannot provide regular screening, and must rely on infrequent screening. A study of cervical cancer screening of 131,746 women in rural India found that a single DNA test reduced the number of advanced cervical cancers and deaths over 8 years, while a single acetic acid examination or a single Pap screening did not. However, the DNA test cost US $30–40, which was unaffordable in many regions, it is time-consuming, and requires a sophisticated laboratory infrastructure. A simple, affordable, and accurate test is being evaluated in China and other countries.[34][35][36] The new test may become available on the market in 2010 at significantly lower cost than current tests.
With HPV testing, there was a 50 percent reduction [37][38] in the number of deaths from cervical cancer compared to unscreened women. Compared to other methods, the research showed the HPV testing reported the fewest false negatives.[39]
Other options
The Bill and Melinda Gates Foundation has funded an eight-year study of a DNA test for the virus that causes cervical cancer. The test manufactured by Qiagen for a low cost per test with results available in only a few hours may allow reduction in use of annual Pap smears. The test has been shown to work "acceptably well" on women who take the swabs themselves rather than allowing a physician to test. This may improve the chances of early diagnosis for women who are unwilling to be screened due to discomfort or modesty.[40]
Visual inspection to detect pre-cancer or cancer
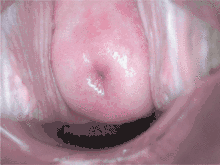
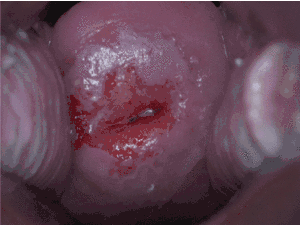
In areas where Pap smear screening is not available or affordable, other methods of testing have been evaluated.
Visual inspection of the cervix, using acetic acid (white vinegar; VIA) or Lugol’s iodine (VILI) to highlight precancerous lesions so they can be viewed with the "naked eye", shifts the identification of precancer from the laboratory to the clinic. Such procedures eliminate the need for laboratories and transport of specimens, require very little equipment and provide women with immediate test results. A range of medical professionals—doctors, nurses, or professional midwives—can effectively perform the procedure, provided they receive adequate training and supervision. As a screening test, VIA may perform as well as or better than cervical cytology in accurately identifying pre-cancerous lesions.[41] This has been demonstrated in various studies where trained physicians and mid-level providers correctly identified between 45% and 79% of women at high risk of developing cervical cancer.[42] By comparison, the sensitivity of cytology has been shown to be between 47 and 62%. Cytology provides higher specificity (fewer false positives) than VIA. Like cytology, one of the limitations of VIA is that results are highly dependent on the accuracy of an individual's interpretation. This means that initial training and on-going quality control are of paramount importance. Increased false positives are particularly important in a screen-and-treat setting, since over-treatment and resulting impairment of fertility is more likely.
VIA can offer significant advantages over Pap in low-resource settings, particularly in terms of increased screening coverage, improved follow-up care and overall program quality. Due to the need for fewer specialized personnel and less infrastructure, training, and equipment, with VIA public health systems can offer cervical cancer screening in more remote (and less equipped) health care settings and can achieve higher coverage. Furthermore, providers can share the results of VIA with patients immediately, making it possible to screen and treat women during the same visit. This helps ensure that follow-up care can be provided on the spot and reduces the number of women who may miss out on treatment because they are not able to return to the clinic at another time. In a "screen and treat" project in Peru, for example, only 9% of women who screened positive failed to receive treatment in the single-visit approach, compared with 44% of women who were lost to treatment using a multi-visit model.[43]
VIA has successfully been paired with cryotherapy, a relatively simple and inexpensive method of treating cervical lesions that can be performed by primary care physicians and mid-level providers.[44]
Visual inspection with acetic acid
Also a promising approach visual inspection with acetic acid (VIA) has to be analyzed if considered for public health initiatives. VIA has shown to have in several studies a low specificity compared to cytology and a high rate of false positives.[45][46][47][48] Entities such as inflammation, cervical condyloma and leukoplakia can give false positive results of VIA test.[49] It also has a low positive predictive value resulting in overdiagnosis and overtreatment. VIA has severe limitations with lesions above the endocervical canal which cannot be visualized; this represents a major problem specially for postmenopausal women where the endocervical junction recedes.[50] There is no permanent record of the test to be reviewed later. Between community centers high variability has been observed, and even in a study of Nigeria of 2013 VIA was not reproducible nor sensitive; this led to discouraging the method in that country.[51]
See also
References
- "Cervical Cancer Screening". medlineplus.gov. Retrieved 2020-04-30.
- "What is cervical screening". National Screening Unit, Government of New Zealand. 27 November 2014.
- Module 13: Levels of Disease Prevention. (2007, April 24). Retrieved March 16, 2014, from Centers for Disease Control and Prevention website: "Archived copy". Archived from the original on 2014-02-26. Retrieved 2015-08-23.CS1 maint: archived copy as title (link)
- Quinn, M; Babb, P; Jones, J; Allen, E (1999). "Effect of screening on incidence of and mortality from cancer of cervix in England: evaluation based on routinely collected statistics". BMJ. 318 (7188): 904–8. doi:10.1136/bmj.318.7188.904. PMC 27810. PMID 10102852.
- World Health Organization (2014). Comprehensive Cervical Cancer Control: A Guide to Essential Practice. WHO.
- "Everything about cervical cancer prevention". www.ecca.info. Retrieved 2015-05-09.
- Arbyn, M; Anttila, A; Jordan, J; Ronco, G; Schenck, U; Segnan, N; Wiener, H; Herbert, A; von Karsa, L (Mar 2010). "European Guidelines for Quality Assurance in Cervical Cancer Screening. Second edition--summary document". Annals of Oncology. 21 (3): 448–58. doi:10.1093/annonc/mdp471. PMC 2826099. PMID 20176693.
- "Cervical screening: programme overview". GOV.UK. Public Health England.
- "SEER Stat Fact Sheets: Cervix Uteri Cancer". Retrieved 8 April 2014.
- Karjane, N; Chelmow, D (June 2013). "New cervical cancer screening guidelines, again". Obstetrics and Gynecology Clinics of North America. 40 (2): 211–23. doi:10.1016/j.ogc.2013.03.001. PMID 23732026.
- Center for Disease Control. "Cervical Cancer Screening Guidelines for Average-Risk Women" (PDF). Retrieved 17 April 2014.
- Committee on Practice, Bulletins—Gynecology (Nov 2012). "ACOG Practice Bulletin Number 131: Screening for cervical cancer". Obstetrics and Gynecology. 120 (5): 1222–38. doi:10.1097/AOG.0b013e318277c92a. PMID 23090560.
- "Cervical cancer screening", Cancer Council Australia, accessed 14 November 2015
- "Screening for Cervical Cancer (2013)" Archived 2015-11-17 at the Wayback Machine, Canadian Task Force for Preventive Health Care, accessed 14 November 2015
- "Cervical Cancer Screening", Cancer Care Ontario, accessed 14 November 2015
- Sankaranarayanan R, Esmy PO, Rajkumar R, Muwonge R, Swaminathan R, Shanthakumari S, Fayette JM, Cherian J (2007). "Effect of visual screening on cervical cancer incidence and mortality in Tamil Nadu, India: a cluster-randomised trial". Lancet. 370 (9585): 398–406. doi:10.1016/S0140-6736(07)61195-7. PMID 17679017. S2CID 44921227.
- Screening: Cervical cancer, US Preventive Services Task Force (accessed 28/01/2011)
- Liquid Based Cytology (LBC), NHS cervical screening programme (accessed 28/03/2011)
- Coste J, Cochand-Priollet B, de Cremoux P, et al. (2003). "Cross sectional study of conventional cervical smear, monolayer cytology, and human papillomavirus DNA testing for cervical cancer screening". BMJ. 326 (7392): 733. doi:10.1136/bmj.326.7392.733. PMC 152633. PMID 12676841. ACP Journal Club
- Ronco G, Cuzick J, Pierotti P, et al. (2007). "Accuracy of liquid based versus conventional cytology: overall results of new technologies for cervical cancer screening randomised controlled trial". BMJ. 335 (7609): 28. doi:10.1136/bmj.39196.740995.BE. PMC 1910655. PMID 17517761.
- Kulasingam SL, Hughes JP, Kiviat NB, et al. (2002). "Evaluation of human papillomavirus testing in primary screening for cervical abnormalities: comparison of sensitivity, specificity, and frequency of referral". JAMA. 288 (14): 1749–57. doi:10.1001/jama.288.14.1749. PMID 12365959.
- Walboomers JM, Jacobs MV, Manos MM (1999). "Human papillomavirus is a necessary cause of invasive cervical cancer worldwide". J. Pathol. 189 (1): 12–9. doi:10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. PMID 10451482.
- Cuschieri KS, Cubie HA, Whitley MW, et al. (2005). "Persistent high risk HPV infection associated with development of cervical neoplasia in a prospective population study". J. Clin. Pathol. 58 (9): 946–50. doi:10.1136/jcp.2004.022863. PMC 1770812. PMID 16126875.
- Cuzick J, Szarewski A, Terry G, Ho L, Hanby A, Maddox P, Anderson M, Kocjan G, Steele ST, Guillebaud J (1995). "Human papillomavirus testing in primary cervical screening". Lancet. 345 (8964): 1533–6. doi:10.1016/s0140-6736(95)91086-7. PMID 7791438.CS1 maint: multiple names: authors list (link)
- "HPV triage and test of cure in the cervical screening programme in England". Public Health England. Retrieved 28 July 2014.
- Cuzick J, Szarewski A, Cubie H, et al. (2003). "Management of women who test positive for high-risk types of human papillomavirus: the HART study". Lancet. 362 (9399): 1871–6. doi:10.1016/S0140-6736(03)14955-0. PMID 14667741. S2CID 26008721.
- Arbyn M, Buntinx F, Van Ranst M, Paraskevaidis E, Martin-Hirsch P, Dillner J (2004). "Virologic versus cytologic triage of women with equivocal Pap smears: a meta-analysis of the accuracy to detect high-grade intraepithelial neoplasia". J. Natl. Cancer Inst. 96 (4): 280–93. doi:10.1093/jnci/djh037. PMID 14970277.
- Colposcopy and Treatment of Cervical Intraepithelial Neoplasia: A Beginner's Manual
- ASCUS-LSIL Traige Study (ALTS) Group (2003). "Results of a randomized trial on the management of cytology interpretations of atypical squamous cells of undetermined significance". Am. J. Obstet. Gynecol. 188 (6): 1383–92. doi:10.1016/S0002-9378(03)00418-6. PMID 12824967.
- Passmore JA, Morroni C, Shapiro S, Williamson AL, Hoffman M (2007). "Papanicolaou smears and cervical inflammatory cytokine responses". J Inflamm (Lond). 4: 8. doi:10.1186/1476-9255-4-8. PMC 1868022. PMID 17456234.
- Naucler P, Ryd W, Törnberg S, et al. (2007). "Human papillomavirus and Papanicolaou tests to screen for cervical cancer". N. Engl. J. Med. 357 (16): 1589–97. doi:10.1056/NEJMoa073204. PMID 17942872.
- Arbyn M, Verdoodt F, Snijders PJ; et al. (2014). "Accuracy of human papillomavirus testing on self-collected versus clinician-collected samples: a meta-analysis". The Lancet Oncology. 15 (2): 172–83. doi:10.1016/S1470-2045(13)70570-9. PMID 24433684.CS1 maint: multiple names: authors list (link)
- Solomon, C. G.; Solomon, M.; Solomon, D. (2013). "Cervical-Cancer Screening with Human Papillomavirus and Cytologic Cotesting". New England Journal of Medicine. 369 (24): 2324–2331. doi:10.1056/NEJMcp1210379. PMID 24328466.
- Sankaranarayanan R, Nene BM, Shastri SS, et al. (April 2009). "HPV screening for cervical cancer in rural India". N. Engl. J. Med. 360 (14): 1385–94. doi:10.1056/NEJMoa0808516. PMID 19339719. S2CID 30179823.
- Emery, Gene (2009-04-01). "Reuters- QIAGEN virus test cuts death from cervical cancer". Reuters.com. Retrieved 2010-08-29.
- Donald G. McNeil Jr. (April 6, 2009). "New DNA Test Outperforms the Pap Smear". The New York Times.
- Chase, Marilyn (2009-04-01). "Bloomberg – Cervical cancer deaths halved by HPV Test, Treatment". Bloomberg.com. Retrieved 2010-08-29.
- "The Guardian – NHS under pressure for new cervical cancer test provision". Sarah Boseley, health editor. London: Guardian. 2009-04-02. Retrieved 2010-08-29.CS1 maint: others (link)
- Sharples, Tiffany (2009-04-02). "Time – HPV Test Screens Best for Cervical Cancer". Time.com. Retrieved 2010-08-29.
- McNeil Jr, Donald G. (2009-04-07). "DNA Test Outperforms Pap Smear". The New York Times. Retrieved 2010-05-21.
- Sherris J, Wittet S, Kleine A, et al. (September 2009). "Evidence-based, alternative cervical cancer screening approaches in low-resource settings". Int Perspect Sex Reprod Health. 35 (3): 147–54. doi:10.1363/3514709. PMID 19805020.
- Sankaranarayanan, R; Gaffikin, L; Jacob, M; Sellors, J; Robles, S (2005). "A critical assessment of screening methods for cervical neoplasia". International Journal of Gynecology & Obstetrics. 89: S4–S12. doi:10.1016/j.ijgo.2005.01.009. ISSN 0020-7292. PMID 15823266.
- Luciani S, Winkler J. Cervical Cancer prevention in Peru: Lessons learned from the TATI demonstration project. Washington, DC: Pan American Health Organization; 2006.
- Gage, J; Ferreccio, Catterina; Gonzales, Miguel; Arroyo, Raul; Huivı́n, Militza; Robles, Sylvia C (2003). "Follow-up care of women with an abnormal cytology in a low-resource setting". Cancer Detection and Prevention. 27 (6): 466–471. doi:10.1016/j.cdp.2003.09.004. ISSN 0361-090X. PMID 14642555.
- Jeronimo J, et al. (Mar 2014). "A multicountry evaluation of careHPV testing, visual inspection with acetic acid, and papanicolaou testing for the detection of cervical cancer". Int J Gynecol Cancer. 24 (3): 576–85. doi:10.1097/igc.0000000000000084. PMC 4047307. PMID 24557438.
- Longatto-Filho A, et al. (Jun 2012). "Performance characteristics of Pap test, VIA, VILI, HR-HPV testing, cervicography, and colposcopy in diagnosis of significant cervical pathology". Virchows Arch. 460 (6): 577–85. doi:10.1007/s00428-012-1242-y. PMID 22562132. S2CID 20361024.
- Labani S et al. CareHPV cervical cancer screening demonstration in a rural population of north India. Eur J Obstet Gynecol Reprod Biol. 2014 May;
- Gravitt, Patti E.; Paul, Proma; Katki, Hormuzd A.; Vendantham, Haripriya; Ramakrishna, Gayatri; Sudula, Mrudula; Kalpana, Basany; Ronnett, Brigitte M.; Vijayaraghavan, K.; Shah, Keerti V. (2010). "Effectiveness of VIA, Pap, and HPV DNA Testing in a Cervical Cancer Screening Program in a Peri-Urban Community in Andhra Pradesh, India". PLOS ONE. 5 (10): e13711. Bibcode:2010PLoSO...513711G. doi:10.1371/journal.pone.0013711. PMC 2965656. PMID 21060889.
- International Agency for Research on Cancer. A practical manual on visual screening for cervical neoplasia. Lyon, France: IARC; 2003.
- Rahatgaonkar W. VIA in cervical cancer screening. Journal of Dental and Medical Sciences (IOSRJDMS) ISSN 2279-0861 Volume 1, Issue 1 (July–August 2012), PP 01-04
- Ajenifuja KO, et al. (Mar 2013). "A population-based study of visual inspection with acetic acid (VIA) for cervical screening in rural Nigeria". Int J Gynecol Cancer. 23 (3): 507–12. doi:10.1097/igc.0b013e318280f395. PMC 3580031. PMID 23354369.