Calicheamicin
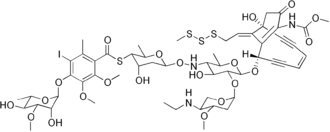
The calicheamicins are a class of enediyne antitumor antibiotics derived from the bacterium Micromonospora echinospora,[1] with calicheamicin γ1 being the most notable.[2] It was isolated originally in the mid-1980s from the chalky soil, or "caliche pits", located in Kerrville, Texas. The sample was collected by a scientist working for Lederle Labs.[3] It is extremely toxic to all cells and, in 2000, a CD33 antigen-targeted immunoconjugate N-acetyl dimethyl hydrazide calicheamicin was developed and marketed as targeted therapy against the non-solid tumor cancer acute myeloid leukemia (AML).[4] A second calicheamicin-linked monoclonal antibody, inotuzumab ozogamicin (marketed as Besponsa) an anti-CD22-directed antibody-drug conjugate, was approved by the U.S. Food and Drug Administration on August 17, 2017, for use in the treatment of adults with relapsed or refractory B-cell precursor acute lymphoblastic leukemia.[5] Calicheamicin γ1 and the related enediyne esperamicin are the two of the most potent antitumor agents known.[6]
 | |
 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C55H74IN3O21S4 | |
| Molar mass | 1368.34 g·mol−1 |
| Hazards | |
| GHS pictograms |   |
| GHS Signal word | Danger |
GHS hazard statements |
H302, H341, H361, H372 |
| P201, P202, P260, P264, P270, P281, P301+312, P308+313, P314, P330, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Mechanism of toxicity
Calicheamicins target DNA and cause strand scission. Calicheamicins bind with DNA in the minor groove, wherein they then undergo a reaction analogous to the Bergman cyclization to generate a diradical species. This diradical, 1,4-didehydrobenzene, then abstracts hydrogen atoms from the deoxyribose (sugar) backbone of DNA, which ultimately leads to strand scission.[7] The specificity of binding of calicheamicin to the minor groove of DNA was demonstrated by Crothers et al. (1999) to be due to the aryltetrasaccharide group of the molecule.[8][9]
Biosynthesis
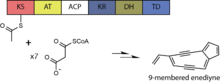
The core metabolic pathway for biosynthesis of this molecule resembles that of other characterized enediyne compounds and occurs via an iterative polyketide synthase (PKS) pathway. This type I PKS loads Acetyl-CoA and then repeatedly adds a total of seven Malonyl-CoAs. The growing polyketide is acted upon by the ketoreductase domain (KR) and dehydratase domain (DH) during each iteration to produce a 15-carbon polyene, which is then processed by accessory enzymes to yield the putative enediyne core of calicheamicin.[10][11][12] Maturation of the polyketide core is anticipated to occur by the action of additional enzymes to provide a calicheamicinone-like intermediate as a substrate for subsequent glycosylation.
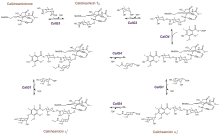
Glycosylation of calicheamicinone requires 4 glycosyltransferases (CalG1-4) and one acyltransferase (CalO4), each recognizing a specific sugar nucleotide or orsellinic acid substrate. Ground-breaking biochemical studies of CalG1-G4 by Thorson and coworkers revealed the reactions catalyzed by these glycosyltransferases to be highly reversible.[13] This was a paradigm shift in the context of glycosyltransferase catalysis and Thorson and coworkers went on to demonstrate this to be a general phenomenon that could be exploited for sugar nucleotide synthesis and 'glycorandomization'.[14] The structures of all four glycosyltransferases were also reported by the same group, revealing a conserved calicheamicin binding motif that coordinates the enediyne backbone thorough interactions with aromatic residues. The catalytic site of CalG1, CalG3, and CalG4 was shown to possess a highly conserved catalytic dyad of histidine and aspartate which promotes nucleophilic attack on the acceptor hydroxyl group of calicheamicin intermediates. Notably, this motif is absent from CalG2, suggesting a different catalytic mechanism in this enzyme.[15]
Resistance
Calicheamicin displays unbiased toxicity to bacteria, fungi, viruses, and eukaryotic cells and organisms, which raises questions as to how the calicheamicin-producing Micromonospora manages not to poison itself. An answer to this question was presented in 2003 when Thorson and coworkers presented the first known example of a "self-sacrifice" resistance mechanism encoded by the gene calC from the calicheamicin biosynthetic gene cluster.[16] In this study, the scientists revealed calicheamicin to cleave the protein CalC site-specifically, destroying both the calicheamicin and the CalC protein, thereby preventing DNA damage. The same group went on to solve the structure of CalC and, more recently, in collaboration with scientists from the Center for Pharmaceutical Research and Innovation (CPRI), discover structural or functional homologs encoded by genes in the calicheamicin gene cluster previously listed as encoding unknown function.[17][18] In this latter study, the authors suggest that CalC homologs may serve in a biosynthetic capacity as the long-sought-after polyketide cyclases required to fold or cyclize early intermediates en route to calicheamicin.
History
It has been proposed that Alexander the Great was poisoned by drinking the water of the river Mavroneri (identified with the mythological River Styx) which is postulated to have been contaminated by this compound. However, toxicologists believe an extensive knowledge of biological chemistry would have been requisite for any application of this poison in antiquity.[19][20]
See also
- Antibody-drug conjugates using calicheamicins as cytotoxic agents:
References
- Maiese, William M; Lechevalier, Mary P.; Lechevalier, Hubert A; Korshalla, Joseph; Kuck, Nydia; Fantini, Amadeo; Wildey, Mary Jo; Thomas, John; Greenstein, Michael (April 1989). "Calicheamicins, a novel family of antitumor antibiotics: taxonomy, fermentation and biological properties". Journal of Antibiotics. 42 (4): 558–63. doi:10.7164/antibiotics.42.558. PMID 2722671.
- Lee, May D.; Manning, Joann K.; Williams, David R.; Kuck, Nydia A.; Testa, Raymond T.; Borders, Donald B. (July 1989). "Calichemicins, a novel family of antitumor antibiotics. 3. Isolation, purification and characterization of calichemicins β1Br, γ1Br, α2I, α3I, β1I, γ1I, and Δ1I". Journal of Antibiotics. 42 (7): 1070–87. doi:10.7164/antibiotics.42.1070. PMID 2753814.
- Total Synthesis and the Creative Process: An Interview with K.C. Nicolaou, Scripps Research Institute
- G.A. Ellestad (2011). "Structural and Conformational Features Relevant to the Anti-Tumor Activity of Calichemicin γ1I". Chirality. 23 (8): 660–671. doi:10.1002/chir.20990. PMID 21800378.
- ASH Clinical News, Volume 4, Number 2, January 2018. Newly Approved Drugs in ALL and NHL: How to Use Them in Practice. pp. 26-27.
- Calicheamicin and Esperamicin are the two most potent antitumor agents known to man Archived 2008-09-21 at the Wayback Machine, Univ Of Georgia, Chem 4500
- S. Walker; R. Landovitz; W.D. Ding; G.A. Ellestad; D. Kahne (1992). "Cleavage behavior of calicheamicin gamma 1 and calicheamicin T". Proc Natl Acad Sci USA. 89 (10): 4608–12. doi:10.1073/pnas.89.10.4608. PMC 49132. PMID 1584797.
- Simkhada D, Oh TJ, Kim EM, Yoo JC, Sohng JK (January 2009). "Cloning and characterization of CalS7 from Micromonospora echinospora sp. calichensis as a glucose-1-phosphate nucleotidyltransferase". Biotechnol. Lett. 31 (1): 147–53. doi:10.1007/s10529-008-9844-9. PMID 18807197.
- Zhang C, Bitto E, Goff RD, Singh S, Bingman CA, Griffith BR, Albermann C, Phillips GN Jr, Thorson JS (Aug 25, 2008). "Biochemical and structural insights of the early glycosylation steps in calicheamicin biosynthesis". Chem. Biol. 15 (8): 842–53. doi:10.1016/j.chembiol.2008.06.011. PMC 2965851. PMID 18721755.
- Horsman, GP; Chen, Y; Thorson, JS; Shen, B (Jun 22, 2010). "Polyketide synthase chemistry does not direct biosynthetic divergence between 9- and 10-membered enediynes". Proc Natl Acad Sci U S A. 107 (25): 11331–5. doi:10.1073/pnas.1003442107. PMC 2895059. PMID 20534556.
- Ahlert, J.; Shepard, E.; Lomovskaya, N.; Zazopoulos, E.; Staffa, A. (2002), "The calicheamicin gene cluster and its iterative type I enediyne", Science, 297 (5584): 1173–6, doi:10.1126/science.1072105, PMID 12183629
- Galm, U; Hager, MH; Van Lanen, SG; Ju, J; Thorson, JS; Shen, B (Feb 2005). "Antitumor antibiotics: bleomycin, enediynes, and mitomycin". Chemical Reviews. 105 (2): 739–58. doi:10.1021/cr030117g. PMID 15700963.
- Zhang, C; Griffith, BR; Fu, Q; Albermann, C; Fu, X; Lee, IK; Li, L; Thorson, JS (Sep 1, 2006). "Exploiting the reversibility of natural product glycosyltransferase-catalyzed reactions". Science. 313 (5791): 1291–4. doi:10.1126/science.1130028. PMID 16946071.
- Gantt, RW; Peltier-Pain, P; Cournoyer, WJ; Thorson, JS (Aug 21, 2011). "Using simple donors to drive the equilibria of glycosyltransferase-catalyzed reactions". Nature Chemical Biology. 7 (10): 685–91. doi:10.1038/nchembio.638. PMC 3177962. PMID 21857660.
- Chang, A.; Singh, S.; Helmich, K. (2011), "Complete set of glycosyltransferase structures in the calicheamicin biosynthetic pathway reveals the origin of regiospecificity", Proceedings of the National Academy of Sciences, 108 (43): 17649–54, doi:10.1073/pnas.1108484108, PMC 3203770, PMID 21987796
- Biggins, JB; Onwueme, KC; Thorson, JS (Sep 12, 2003). "Resistance to enediyne antitumor antibiotics by CalC self-sacrifice". Science. 301 (5639): 1537–41. doi:10.1126/science.1086695. PMID 12970566.
- Singh, S; Hager, MH; Zhang, C; Griffith, BR; Lee, MS; Hallenga, K; Markley, JL; Thorson, JS (Aug 22, 2006). "Structural insight into the self-sacrifice mechanism of enediyne resistance". ACS Chemical Biology. 1 (7): 451–60. doi:10.1021/cb6002898. PMID 17168523.
- Elshahawi, SI; Ramelot, TA; Seetharaman, J; Chen, J; Singh, S; Yang, Y; Pederson, K; Kharel, MK; Xiao, R; Lew, S; Yennamalli, RM; Miller, MD; Wang, F; Tong, L; Montelione, GT; Kennedy, MA; Bingman, CA; Zhu, H; Phillips GN, Jr; Thorson, JS (Aug 13, 2014). "Structure-Guided Functional Characterization of Enediyne Self-Sacrifice Resistance Proteins, CalU16 and CalU19". ACS Chemical Biology. 9 (10): 2347–58. doi:10.1021/cb500327m. PMC 4201346. PMID 25079510.
- Nick Squires (August 4, 2010). "Alexander the Great poisoned by the River Styx.html". Telegraph.
- Rossella Lorenzi (16 Jul 2010). "Alexander the Great killed by toxic bacteria?". Discovery News.