Benz(a)anthracene
Benz[a]anthracene or benzo[a]anthracene is a polycyclic aromatic hydrocarbon with the chemical formula C18H12.[2] It is produced during incomplete combustion of organic matter.
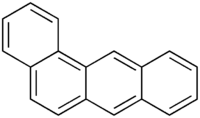 | |
anthracene_molecule_ball.png) | |
| Names | |
|---|---|
| Preferred IUPAC name
Tetraphene[1] | |
| Other names
Benz[a]anthracene Benzanthracene Benzanthrene 1,2-Benzanthracene Benzo[b]phenanthrene Tetracyclo[8.8.0.02,7.012,17]octadeca-1,3,5,7,9,11,13,15,17-nonaene | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.000.255 |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C18H12 | |
| Molar mass | 228.294 g·mol−1 |
| Appearance | White solid |
| Density | 1.19 g/cm3 |
| Melting point | 158 °C (316 °F; 431 K) |
| Boiling point | 438 °C (820 °F; 711 K) |
| Hazards | |
| Flash point | 209.1 °C (408.4 °F; 482.2 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Benz[a]anthracene is one of carcinogenic constituents of tobacco smoke.[3]
See also
- Tetracene, also known as benz[b]anthracene
References
- "Front Matter". Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 208. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- "Benzo[a]anthracene [MAK Value Documentation, 2012]". The MAK-Collection for Occupational Health and Safety. Wiley-VCH Verlag GmbH & Co. KGaA. 2002. pp. 231–242. doi:10.1002/3527600418.mb5655e0027. ISBN 9783527600410.
- Talhout, Reinskje; Schulz, Thomas; Florek, Ewa; Van Benthem, Jan; Wester, Piet; Opperhuizen, Antoon (2011). "Hazardous Compounds in Tobacco Smoke". International Journal of Environmental Research and Public Health. 8 (12): 613–628. doi:10.3390/ijerph8020613. ISSN 1660-4601. PMC 3084482. PMID 21556207.
External links
- Toxic Substances Portal - Polycyclic Aromatic Hydrocarbons A resource summarizing many toxicological aspects of benzanthracene and other polycyclic aromatic hydrocarbons.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.