Fluoranthene
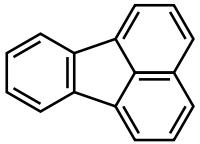
Fluoranthene is a polycyclic aromatic hydrocarbon (PAH). The molecule can be viewed as the fusion of naphthalene and benzene unit connected by a five-membered ring. Although samples are often pale yellow, the compound is colorless. It is soluble in nonpolar organic solvents.[2] It is a member of the class of PAHs known as non-alternant PAHs because it has rings other than those with six carbon atoms. It is a structural isomer of the alternant PAH pyrene. It is not as thermodynamically stable as pyrene. Its name is derived from its fluorescence under UV light.
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Fluoranthene[1] | |
| Other names
Benzo[jk]fluorene Tetracyclo[7.6.1.05,16.010,15]hexadeca-1,3,5,7,9(16),10,12,14-octaene | |
| Identifiers | |
3D model (JSmol) |
|
| 1907918 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.005.376 |
| EC Number |
|
| 262216 | |
| KEGG | |
PubChem CID |
|
| UNII | |
| UN number | 1325, 3082 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C16H10 | |
| Molar mass | 202.256 g·mol−1 |
| Appearance | Yellow to green needles |
| Density | 1.252 g/cm3 (0 °C), solid |
| Melting point | 110.8 °C (231.4 °F; 383.9 K) |
| Boiling point | 375 °C (707 °F; 648 K) |
| 265 μg/l (25 °C) | |
| -138.0·10−6 cm3/mol | |
| Viscosity | 0.652 cP at 20 °C |
| Structure | |
| Planar | |
| 0.34 D | |
| Hazards | |
| Flash point | 210 °C (410 °F; 483 K) |
| Related compounds | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Occurrence
Coal tar
Fluoranthene was originally isolated from coal tar pitch. It is still obtained from the high boiling fraction of coal tar, representing a few percent by weight.[2]
Pollutant
Fluoranthene is found in many combustion products, along with other PAHs. Its presence is an indicator of less efficient or lower-temperature combustion, since non-alternant PAHs are less preferred in formation than alternant PAHs. Fluoranthene is one of the U.S. Environmental Protection Agency's 16 priority pollutant PAHs. Fluoranthene has been classified by the International Agency for Research on Cancer as a group 3 carcinogen, "not classifiable as to its carcinogenicity to humans"[1] , however it was found to possess carcinogenic properties in case of newborn mice according to short-term lung tumor assay (Busby et al., 1984).[3] In 2019, fluoranthene was added to the Candidate List of Substances of Very High Concern (SVHCs) due to its persistent, bioaccumulative and toxic (PBT) and very persistent and very bioaccumulative (vPvB) properties.[4]
References
- Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. pp. 206, 503. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- Karl Griesbaum, Arno Behr, Dieter Biedenkapp, Heinz-Werner Voges, Dorothea Garbe, Christian Paetz, Gerd Collin, Dieter Mayer, Hartmut Höke “Hydrocarbons” in Ullmann's Encyclopedia of Industrial Chemistry 2002 Wiley-VCH, Weinheim. doi:10.1002/14356007.a13_227
- "Archived copy" (PDF). Archived from the original (PDF) on 2012-09-11. Retrieved 2012-07-30.CS1 maint: archived copy as title (link)
- "Six new substances added to the Candidate List ECHA/PR/19/01". Retrieved 2019-01-17.