Benvitimod
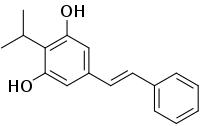
Benvitimod (also known as tapinarof or 3,5-dihydroxy-4-isopropyl-trans-stilbene) is a bacterial stilbenoid produced in Photorhabdus bacterial symbionts of Heterorhabditis nematodes. It is a product of an alternative ketosynthase-directed stilbenoids biosynthesis pathway. It is derived from the condensation of two β-ketoacyl thioesters .[1] It is produced by the Photorhabdus luminescens bacterial symbiont species of the entomopathogenic nematode, Heterorhabditis megidis. Experiments with infected larvae of Galleria mellonella, the wax moth, support the hypothesis that the compound has antibiotic properties that help minimize competition from other microorganisms and prevents the putrefaction of the nematode-infected insect cadaver.[2]
_Poinar%2C_1975.jpg)
 | |
| Names | |
|---|---|
| IUPAC name
(E)-5-(2-Phenylethenyl)-2-propan-2-ylbenzene-1,3-diol | |
Other names
| |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C17H18O2 | |
| Molar mass | 254.329 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Medical research
Benvitimod is being studied in clinical trials for the treatment of plaque psoriasis.[3]
See also
- Pinosylvin, a molecule produced in pines that does not bear the isopropyl alkylation.
References
- Joyce SA; Brachmann AO; Glazer I; Lango L; Schwär G; Clarke DJ; Bode HB (2008). "Bacterial biosynthesis of a multipotent stilbene". Angew Chem Int Ed Engl. 47 (10): 1942–5. CiteSeerX 10.1.1.603.247. doi:10.1002/anie.200705148. PMID 18236486.
- Hu, K; Webster, JM (2000). "Antibiotic production in relation to bacterial growth and nematode development in Photorhabdus--Heterorhabditis infected Galleria mellonella larvae". FEMS Microbiology Letters. 189 (2): 219–23. doi:10.1111/j.1574-6968.2000.tb09234.x. PMID 10930742.
- "New Topical for Mild to Moderate Psoriasis in the Works". Medscape. March 5, 2017.