Bone morphogenetic protein 4
Bone morphogenetic protein 4 is a protein that in humans is encoded by BMP4 gene.[4][5] BMP4 is found on chromosome 14q22-q23
BMP4 is a member of the bone morphogenetic protein family which is part of the transforming growth factor-beta superfamily. The superfamily includes large families of growth and differentiation factors. BMP4 is highly conserved evolutionarily. BMP4 is found in early embryonic development in the ventral marginal zone and in the eye, heart blood and otic vesicle.[6]
Discovery
Bone morphogenetic proteins were originally identified by an ability of demineralized bone extract to induce endochondral osteogenesis in vivo in an extraskeletal site.
Function
BMP4 is a polypeptide belonging to the TGF-β superfamily of proteins. It, like other bone morphogenetic proteins, is involved in bone and cartilage development, specifically tooth and limb development and fracture repair. This particular family member plays an important role in the onset of endochondral bone formation in humans. It has been shown to be involved in muscle development, bone mineralization, and ureteric bud development.
In human embryonic development, BMP4 is a critical signaling molecule required for the early differentiation of the embryo and establishing of a dorsal-ventral axis. BMP4 is secreted from the dorsal portion of the notochord, and it acts in concert with sonic hedgehog (released from the ventral portion of the notochord) to establish a dorsal-ventral axis for the differentiation of later structures.
BMP4 stimulates differentiation of overlying ectodermal tissue.
Bone morphogenetic proteins are known to stimulate bone formation in adult animals. This is thought that inducing osteoblastic commitment and differentiation of stem cells such as mesenchymal stem cells.BMPs are known to play a large role in embryonic development. In the embryo BMP4 helps establish dorsal-ventral axis formation in xenopus through inducing ventral mesoderm. In mice targets inactivation of BMP4 disrupts mesoderm from forming. As well establishes dorsal-ventral patterning of the developing neural tube with the help of BMP7, and inducing dorsal characters.
BMP4 also limits the extent to which neural differentiation in xenopus embryos occurs by inducing epidermis. They can aid in inducing the lateral characteristics in somites. Somites are required for the development of things such as muscles within limbs. BMP4 helps in the patterning of the developing head though inducing apoptosis of the neural crest cells; this is done in the hindbrain.[7]
In adult, BMP4 is important for the neurogenesis (i.e., the generation of new neurons) that occurs throughout life in two neurogenic niches of the brain, the dentate gyrus of the hippocampus and the subventricular zone (SVZ) adjacent to lateral ventricles. In these niches new neurons are continuously generated from stem cells. In fact it has been shown that in the dentate gyrus BMP4 maintains neural stem cells in quiescence, thus preventing the depletion of the pool of stem cells.[8] In the SVZ , BMP-mediated signaling via Smad4 is required to initiate neurogenesis from adult neural stem cells and suppress the alternative fate of oligodendrogliogenesis.[9] Moreover, it has been shown that in the SVZ BMP4 has a prodifferentiative effect, since it rescues a defect of terminal differentiation in SVZ neurospheres where the gene Tis21/BTG2 - required for terminal differentiation - has been deleted.[10] Tis21 is a positive regulator of BMP4 expression in the SVZ.[10]
BMP4 is important for bone and cartilage metabolism. The BMP4 signaling has been found in formation of early mesoderm and germ cells. Limb bud regulation and development of the lungs, liver, teeth and facial mesenchyme cells are other important functions attributed to BMP4 signaling.[11] Digit formation is influenced by BMP4, along with other BMP signals. The interdigital mesenchyme exhibits BMP4, which prevents apoptosis of the region.[12] Tooth formation relies on BMP4 expression, which induces Msx 1 and 2. These transcription factors turn the forming tooth to become and incisor.
BMP4 also plays important roles in adipose tissue: it is essential for white adipogenesis, and promotes adipocyte differentiation.[13] Additionally, it is also important for brown fat, where it induces UCP1, related to non-shivering thermogenesis.[13]
BMP4 secretion helps cause differentiation of the ureteric bud into the ureter.[14]
BMP4 antagonizes organizer tissue and is expressed in early development in ectoderm and mesoderm tissue. Upon gastrulation, the transcription of BMP4 is limited to the ventrolateral marginal zone due to inhibition from the dorsalizing side of the developing embryo. BMP4 aids in ventralizing mesoderm, which guides the dorsal-ventral axis formation. In Xenopus BMP4 has been found to aid in formation of blood and blood islands.[15]
BMP4, initially expressed in the epidermis, is found in the roof plate during formation of the neural tube. A gradient of BMP signaling is found in opposition to a Sonic hedgehog, Shh, gradient. This expression of BMP4 patterns the dorsal neurons.[16]
BMP4, in conjunction with FGF2, promote differentiation of stem cells to mesodermal lineages. After differentiation, BMP4 and FGF2 treated cells generally produces higher amounts of osteogenic and chondorgenic differentiation than untreated stem cells.[17] Also in conjunction with FGF2 it can produce progenitor thyroid cells from pluripotent stem cells in mice and humans.[18]
BMP4 has been shown to induce the expression of the Msx gene family, which is believed to be part of cartilage formation from somitic mesoderm.[19]
BMP4, a paracrine growth factor, has been found in rat ovaries. BMP4, in conjunction with BMP7, regulate early ovarian follicle development and primordial-to-primary follicle transition. In addition, inhibition of BMP4 with antibodies has been shown to decrease overall ovary size. These results indicate that BMP4 may aid in survival and prevention of apoptosis in oocytes.[11]
In birds, BMP4 has been shown to influence the beak size of Darwin's finches. Low amounts of BMP4 are correlated with low beak depths and widths. Conversely, high BMP4 expression makes high beak depths and widths. The genetic regulation of BMP4 provides the foundation for natural selection in bird beaks.[20]
Protein structure
Yielding an active carboxy-terminal peptide of 116 residues, human bmp4 is initially synthesized as a forty percent residue preproprotein which is cleaved post translationally. BMP4 has seven residues which are conserved and glycosylated.[21] The monomers are held with disulphide bridges and 3 pairs of cysteine amino acids. This conformation is called a “cystine knot”. BMP4 can form homodimers or heterodimers with similar BMPS. One example of this is BMP7. This ability to form homodimers or heterodimers gives the ability to have greater osteoinductive activity than just bmp4 alone.[22] Not much is known yet about how BMPS interact with the extracellular matrix. As well little is known about the pathways which then degrade BMP4.
Inhibition
Inhibition of the BMP4 signal (by chordin, noggin, or follistatin) causes the ectoderm to differentiate into the neural plate. If these cells also receive signals from FGF, they will differentiate into the spinal cord; in the absence of FGF the cells become brain tissue.
While overexpression of BMP4 expression can lead to ventralization, inhibition with a dominant negative may result in complete dorsalization of the embryo or the formation of two axises.[23]
It is important to note that mice in which BMP4 was inactivated usually died during gastrulation. It is thought that inactivation of human BMP4 would likely have the same effect. However, mutations which are subtle in humans could also have subtle effects phenotypically.
Isoforms
Alternative splicing in the 5' untranslated region of this gene has been described and three variants are described, all encoding an identical protein.[24]
Molecular mechanisms
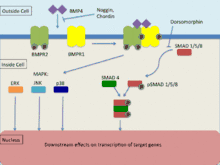
BMP4, as a member of the transforming growth factor-β (TGF-β) family binds to 2 different types of serine-threonine kinase receptors known as BMPR1 and BMPR2.[25] Signal transduction via these receptors occurs via Smad and map kinase pathways to effect transcription of its target genes. In order for signal transduction to occur, both receptors must be functional. BMP is able to bind to BMPR2 without BMPR1 however, the affinity significantly increases in the presence of both receptors. BMPR1 is transphosphorylated via BMPR2 which induces downstream signalling within the cell, affecting transcription.[25]
Smad signaling pathway
TGF-β family receptors most commonly use the Smad signaling pathway to tranduce signals.[25] Type 2 receptors are responsible for activating type 1 receptors where their function involves the phosphorylation of R-Smads (Smad-1, Smad-5, Smad-8). Upon phosphorylation, formation of an R-SMAD complex in conjunction with common-partner Smad (co-Smad) occurs where it migrates to the nucleus. This signaling pathway is regulated by the small molecule inhibitor known as dorsomorphin which prevents the downstream effects of R-smads.[25]
Map kinase (MAPK) signaling pathways
Mitogen activated protein kinases (MAPK) undergo phosphorylation via a signaling cascade where MAPKKK phosphorylates and activates MAPKK and MAPKK phosphorylates and activates MAPK which then induces an intracellular response.[26] Activation of MAPKKK is through the interaction of mainly GTPases or another group of protein kinases. TGF-β receptors induce the MAPK signaling pathways of ERK, JNK and p38.[26] BMP4 is also known to activate the ERK, JNK and p38 MAPK signalling pathways whilst have been found to act independently of Smad signaling pathways, are mostly active in conjunction with Smad.[27] The activation of the ERK and JNK pathways acts to phosphorylate Smad and therefore regulate its activation. In addition to this, MAPK pathways may be able to directly affect Smad-interacting transcription factors via a JNK or p38 substrate that induces convergence of the two signaling pathways. This convergence is noted to consist mainly of cooperative behavior however, there is evidence to suggest that they may at times counteract each other. Furthermore, the balance that exists between the direct activation of these signaling pathways has a significant effect on TGF-β induced cellular responses.[27]
Clinical significance
Increase in expression of BMP4 has been associated with a variety of bone diseases, including the heritable disorder Fibrodysplasia Ossificans Progressiva.[28]
There is strong evidence from sequencing studies of candidate genes involved in clefting that mutations in the bone morphogenetic protein 4 (BMP4) gene may be associated in the pathogenesis of cleft lip and palate.[29]
Eye development
Eyes are essential for organisms, especially terrestrial vertebrates, to observe prey and obstacles; this is critical for their survival. The formation of the eyes starts as optic vesicles and lens derived from the neuroectoderm. Bone morphogenic proteins are known to stimulate eye lens formation. During early development of eyes, the formation of the optic vesicle is essential in Mice and BMP4 expressed strongly in the optic vesicle and weakly in the surrounding mesenchyme and surface ectoderm. This concentration gradient of BMP4 in optic vesicle is critical for lens induction. Researcher, Dr. Furuta and Dr. Hogan found out that if they did a laser mutation on mice embryos and causing a BMP4 homozygous null mutation, this embryo will not develop the lens. They also did an in situ hybridization of the BMP4 gene showing green color and Sox2 gene in red which they thought it was involved in the lens formation as well. After they did these two in situ hybridizations in the mice embryos, they found that both green and red colors are found in the optic vesicle of the mice embryos. This indicated that BMP4 and Sox2 are expressed in the right place at the right time of the optic vesicle and prove that they have some essential functions for the lens induction. Furthermore, they did a follow-up experiment that by injecting BMP4 into the BMP4 homozygous mutant embryos rescued the lens formation (12). This indicated that BMP4 is definitely required for lens formation. However, researchers also found that some of the mutated mice cannot be rescued. They later found that those mutants lacked of Msx 2 which is activated by BMP4. The mechanism they predicted was that BMP4 will active Msx 2 in the optic vesicle and concentration combination of BMP4 and Msx2 together active Sox2 and the Sox2 is essential for lens differentiation.[30]
Injection of Noggin into lens fiber cells in mice significantly reduces the BMP4 proteins in the cells. This indicates that Noggin is sufficient to inhibit the production of BMP4. Moreover, another inhibitor protein, Alk6 was found that blocked the BMP4 from activating the Msx2 which stopped lens differentiation .[31] However, there are still a lot of unknown about the mechanism of inhibition on BMP4 and downstream regulation of Sox2. In the future, researchers are aiming to find out a more complete pathway of whole eye development and hoping one day, they can find a way to cure some genetic caused eye diseases.
Hair loss
Hair loss or known as alopecia is caused from the changing of hair follicle morphology and hair follicle cycling in an abnormal fashion.[32] The cycles of hair follicles are that of growth, or anagen, regression or catagen, and rest or telogen.[33] In mammals reciprocal epithelial and mesynchymal interactions control the development of hair. Genes such as BMP4 and BMP2 are both active within the precursors of the hair shaft. Specifically BMP4 is found in the dermal papilla. BMP4 is part of the signaling network which controls the development of hair. It is needed for the induction of biochemical pathways and signaling for regulating the differentiation of the hair shaft in the anagen hair follicle. This is done through controlling the expression of the transcription factors which regulate hair differentiation. It is still unclear however where BMPs act within the genetic network. The signaling of bmp4 may potentially control expression of terminal differentiation molecules such as keratins. Other regulators have been shown to control hair follicle development as well. HOXC13 and FOXN1 are considered important regulators because loss-of-function experiments show impaired hair shaft differentiation that doesn’t interfere in the hair follicle formation.[34]
When BMP4 is expressed ectopically, within transgenic mice the hair follicle outer root sheath (ORS) the proliferation of the cell matrix is inhibited. BMP4 also activates hair keratin gene expression noting that BMP4 is important in the differentiation of the hair shaft. Noggin, a known inhibitor of BMP4, is found within the matrix cells of the hair bulb. Other important factors to consider in the development of hair is the expression of Shh (sonic hedgehog), BMP7, BMP2, WNT, and β-catenin as these are required in early stage morphogenesis.[35]
Other genes which can inhibit or interact with BMP4 are noggin, follistatin, gremlin, which is all expressed in the developing hair follicles.[36] In mice in which noggin is lacking, there are fewer hair follicles than on a normal mouse and the development of the follicle is inhibited. In chick embryos it is shown that ectopically expressed noggin produces enlarged follicles, and BMP4 signaling shows repressed placode fate in nearby cells.[22] Noggin has also been shown during in vivo experiments to induce hair growth in post natal skin.[37]
BMP4 is an important component of the biological pathways that involved regulating hair shaft differentiation within the anagen hair follicle. The strongest levels of expressed BMP4 are found within the medulla, hair shaft cells, distal hair matrix, and potential precursors of the cuticle. The two main methods which BMP4 inhibit expression of hair is through restricting growth factor expression in the hair matrix and antagonism between growth and differentiation signaling.[35]
Pathways that regulate hair follicle formation and hair growth are key in developing therapeutic methods for hair loss conditions. Such conditions include the development of new follicles, changing the shape of characteristics of existing follicles, and the altering of hair growth in existing hair follicles. Furthermore, BMP4 and the pathway through which it works may provide therapeutic targets for the prevention of hair loss.[33]
References
- GRCm38: Ensembl release 89: ENSMUSG00000021835 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- van den Wijngaard A, Weghuis DO, Boersma CJ, van Zoelen EJ, Geurts van Kessel A, Olijve W (November 1995). "Fine mapping of the human bone morphogenetic protein-4 gene (BMP4) to chromosome 14q22-q23 by in situ hybridization". Genomics. 27 (3): 559–60. doi:10.1006/geno.1995.1096. hdl:2066/22049. PMID 7558046.
- Oida S, Iimura T, Maruoka Y, Takeda K, Sasaki S (November 1995). "Cloning and sequence of bone morphogenetic protein 4 (BMP-4) from a human placental cDNA library". DNA Seq. 5 (5): 273–5. doi:10.3109/10425179509030980. PMID 7579580.
- Knöchel S, Dillinger K, Köster M, Knöchel W (November 2001). "Structure and expression of Xenopus tropicalis BMP-2 and BMP-4 genes". Mech. Dev. 109 (1): 79–82. doi:10.1016/S0925-4773(01)00506-8. PMID 11677055.
- Graham et al., 1994
- Mira H, Andreu Z, Suh H, Lie DC, Jessberger S, Consiglio A, San Emeterio J, Hortigüela R, Marqués-Torrejón MA, Nakashima K, Colak D, Götz M, Fariñas I, Gage FH (2010). "Signaling through BMPR-IA regulates quiescence and long-term activity of neural stem cells in the adult hippocampus". Cell Stem Cell. 7 (1): 78–89. doi:10.1016/j.stem.2010.04.016. PMID 20621052.
- Colak D, Mori T, Brill MS, Pfeifer A, Falk S, Deng C, Monteiro R, Mummery C, Sommer L, Götz M (2008). "Adult neurogenesis requires Smad4-mediated bone morphogenic protein signaling in stem cells". The Journal of Neuroscience. 28 (2): 434–446. doi:10.1523/JNEUROSCI.4374-07.2008. PMC 6670509. PMID 18184786.
- Farioli-Vecchioli S, Ceccarelli M, Saraulli D, Micheli L, Cannas S, D'Alessandro F, Scardigli R, Leonardi L, Cinà I, Costanzi M, Mattera A, Cestari V, Tirone F (2014). "Tis21 is required for adult neurogenesis in the subventricular zone and for olfactory behavior regulating cyclins, BMP4, Hes1/5 and Ids". Front Cell Neurosci. 8: 98. doi:10.3389/fncel.2014.00098. PMC 3977348. PMID 24744701.
- Nilsson EE, Skinner MK (October 2003). "Bone morphogenetic protein-4 acts as an ovarian follicle survival factor and promotes primordial follicle development". Biol. Reprod. 69 (4): 1265–72. doi:10.1095/biolreprod.103.018671. PMID 12801979.
- Arteaga-Solis E, Gayraud B, Lee SY, Shum L, Sakai L, Ramirez F (July 2001). "Regulation of limb patterning by extracellular microfibrils". J. Cell Biol. 154 (2): 275–81. doi:10.1083/jcb.200105046. PMC 2150751. PMID 11470817.
- Blázquez-Medela AM, Jumabay M, Boström KI (May 2019). "Beyond the bone: Bone morphogenetic protein signaling in adipose tissue". Obesity Reviews. 20 (5): 648–658. doi:10.1111/obr.12822. PMC 6447448. PMID 30609449.
- Miyazaki Y, Oshima K, Fogo A, Ichikawa I (March 2003). "Evidence that bone morphogenetic protein 4 has multiple biological functions during kidney and urinary tract development". Kidney Int. 63 (3): 835–44. doi:10.1046/j.1523-1755.2003.00834.x. PMID 12631064.
- Hemmati-Brivanlou A, Thomsen GH (1995). "Ventral mesodermal patterning in Xenopus embryos: expression patterns and activities of BMP-2 and BMP-4". Dev. Genet. 17 (1): 78–89. doi:10.1002/dvg.1020170109. PMID 7554498.
- Selleck MA, García-Castro MI, Artinger KB, Bronner-Fraser M (December 1998). "Effects of Shh and Noggin on neural crest formation demonstrate that BMP is required in the neural tube but not ectoderm". Development. 125 (24): 4919–30. PMID 9811576.
- Lee TJ, Jang J, Kang S, Jin M, Shin H, Kim DW, Kim BS (January 2013). "Enhancement of osteogenic and chondrogenic differentiation of human embryonic stem cells by mesodermal lineage induction with BMP-4 and FGF2 treatment". Biochem. Biophys. Res. Commun. 430 (2): 793–7. doi:10.1016/j.bbrc.2012.11.067. PMID 23206696.
- Kurmann AA, Serra M, Hawkins F, Rankin SA, Mori M, Astapova I, Ullas S, Lin S, Bilodeau M, Rossant J, Jean JC, Ikonomou L, Deterding RR, Shannon JM, Zorn AM, Hollenberg AN, Kotton DN (November 2015). "Regeneration of Thyroid Function by Transplantation of Differentiated Pluripotent Stem Cells". Cell Stem Cell. 17 (5): 527–42. doi:10.1016/j.stem.2015.09.004. PMC 4666682. PMID 26593959.
- Watanabe Y, Le Douarin NM (June 1996). "A role for BMP-4 in the development of subcutaneous cartilage". Mech. Dev. 57 (1): 69–78. doi:10.1016/0925-4773(96)00534-5. PMID 8817454.
- Abzhanov A, Protas M, Grant BR, Grant PR, Tabin CJ (September 2004). "Bmp4 and morphological variation of beaks in Darwin's finches". Science. 305 (5689): 1462–5. Bibcode:2004Sci...305.1462A. doi:10.1126/science.1098095. PMID 15353802.
- Aono A, Hazama M, Notoya K, Taketomi S, Yamasaki H, Tsukuda R, Sasaki S, Fujisawa Y (May 1995). "Potent ectopic bone-inducing activity of bone morphogenetic protein-4/7 heterodimer". Biochem. Biophys. Res. Commun. 210 (3): 670–7. doi:10.1006/bbrc.1995.1712. PMID 7763240.
- Botchkarev VA, Botchkareva NV, Roth W, Nakamura M, Chen LH, Herzog W, Lindner G, McMahon JA, Peters C, Lauster R, McMahon AP, Paus R (July 1999). "Noggin is a mesenchymally derived stimulator of hair-follicle induction". Nat. Cell Biol. 1 (3): 158–64. doi:10.1038/11078. PMID 10559902.
- Metz A, Knöchel S, Büchler P, Köster M, Knöchel W (June 1998). "Structural and functional analysis of the BMP-4 promoter in early embryos of Xenopus laevis". Mech. Dev. 74 (1–2): 29–39. doi:10.1016/S0925-4773(98)00059-8. PMID 9651472.
- "Entrez Gene: BMP4 bone morphogenetic protein 4".
- Miyazono K, Kamiya Y, Morikawa M (January 2010). "Bone morphogenetic protein receptors and signal transduction". J. Biochem. 147 (1): 35–51. doi:10.1093/jb/mvp148. PMID 19762341.
- Cell Signaling Technology, Inc. "Mitogen-Activated Protein Kinase Cascades". Retrieved 17 November 2012.
- Derynck R, Zhang YE (October 2003). "Smad-dependent and Smad-independent pathways in TGF-beta family signaling". Nature. 425 (6958): 577–84. Bibcode:2003Natur.425..577D. doi:10.1038/nature02006. PMID 14534577.
- Kan L, Hu M, Gomes WA, Kessler JA (October 2004). "Transgenic Mice Overexpressing BMP4 Develop a Fibrodysplasia Ossificans Progressiva (FOP)-Like Phenotype". Am. J. Pathol. 165 (4): 1107–15. doi:10.1016/S0002-9440(10)63372-X. PMC 1618644. PMID 15466378.
- Dixon MJ, Marazita ML, Beaty TH, Murray JC (March 2011). "Cleft lip and palate: understanding genetic and environmental influences". Nat. Rev. Genet. 12 (3): 167–78. doi:10.1038/nrg2933. PMC 3086810. PMID 21331089.
- Furuta Y, Hogan BL (December 1998). "BMP4 is essential for lens induction in the mouse embryo". Genes Dev. 12 (23): 3764–75. doi:10.1101/gad.12.23.3764. PMC 317259. PMID 9851982.
- Faber SC, Robinson ML, Makarenkova HP, Lang RA (August 2002). "Bmp signaling is required for development of primary lens fiber cells". Development. 129 (15): 3727–37. PMID 12117821.
- Cotsarelis G, Millar SE (July 2001). "Towards a molecular understanding of hair loss and its treatment". Trends Mol Med. 7 (7): 293–301. doi:10.1016/S1471-4914(01)02027-5. PMID 11425637.
- Millar SE (February 2002). "Molecular mechanisms regulating hair follicle development". J. Invest. Dermatol. 118 (2): 216–25. doi:10.1046/j.0022-202x.2001.01670.x. PMID 11841536.
- Kulessa H, Turk G, Hogan BL (December 2000). "Inhibition of Bmp signaling affects growth and differentiation in the anagen hair follicle". EMBO J. 19 (24): 6664–74. doi:10.1093/emboj/19.24.6664. PMC 305899. PMID 11118201.
- Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W (May 2001). "beta-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin". Cell. 105 (4): 533–45. doi:10.1016/S0092-8674(01)00336-1. PMID 11371349.
- Feijen A, Goumans MJ, van den Eijnden-van Raaij AJ (December 1994). "Expression of activin subunits, activin receptors and follistatin in postimplantation mouse embryos suggests specific developmental functions for different activins". Development. 120 (12): 3621–37. PMID 7821227.
- Botchkarev VA, Botchkareva NV, Nakamura M, Huber O, Funa K, Lauster R, Paus R, Gilchrest BA (October 2001). "Noggin is required for induction of the hair follicle growth phase in postnatal skin". FASEB J. 15 (12): 2205–14. doi:10.1096/fj.01-0207com. PMID 11641247. S2CID 10236217.
Further reading
- Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, Hewick RM, Wang EA (1989). "Novel regulators of bone formation: molecular clones and activities". Science. 242 (4885): 1528–34. doi:10.1126/science.3201241. PMID 3201241.
- Rosenzweig BL, Imamura T, Okadome T, Cox GN, Yamashita H, ten Dijke P, Heldin CH, Miyazono K (1995). "Cloning and characterization of a human type II receptor for bone morphogenetic proteins". Proc. Natl. Acad. Sci. U.S.A. 92 (17): 7632–6. Bibcode:1995PNAS...92.7632R. doi:10.1073/pnas.92.17.7632. PMC 41199. PMID 7644468.
- Nohno T, Ishikawa T, Saito T, Hosokawa K, Noji S, Wolsing DH, Rosenbaum JS (1995). "Identification of a human type II receptor for bone morphogenetic protein-4 that forms differential heteromeric complexes with bone morphogenetic protein type I receptors". J. Biol. Chem. 270 (38): 22522–6. doi:10.1074/jbc.270.38.22522. PMID 7673243.
- Yamaji N, Celeste AJ, Thies RS, Song JJ, Bernier SM, Goltzman D, Lyons KM, Nove J, Rosen V, Wozney JM (1995). "A mammalian serine/threonine kinase receptor specifically binds BMP-2 and BMP-4". Biochem. Biophys. Res. Commun. 205 (3): 1944–51. doi:10.1006/bbrc.1994.2898. PMID 7811286.
- Harris SE, Harris MA, Mahy P, Wozney J, Feng JQ, Mundy GR (1994). "Expression of bone morphogenetic protein messenger RNAs by normal rat and human prostate and prostate cancer cells". Prostate. 24 (4): 204–11. doi:10.1002/pros.2990240406. PMID 8146069.
- van den Wijngaard A, van Kraay M, van Zoelen EJ, Olijve W, Boersma CJ (1996). "Genomic organization of the human bone morphogenetic protein-4 gene: molecular basis for multiple transcripts". Biochem. Biophys. Res. Commun. 219 (3): 789–94. doi:10.1006/bbrc.1996.0312. hdl:2066/23948. PMID 8645259.
- Nishitoh H, Ichijo H, Kimura M, Matsumoto T, Makishima F, Yamaguchi A, Yamashita H, Enomoto S, Miyazono K (1996). "Identification of type I and type II serine/threonine kinase receptors for growth/differentiation factor-5". J. Biol. Chem. 271 (35): 21345–52. doi:10.1074/jbc.271.35.21345. PMID 8702914.
- Bonaldo MF, Lennon G, Soares MB (1997). "Normalization and subtraction: two approaches to facilitate gene discovery". Genome Res. 6 (9): 791–806. doi:10.1101/gr.6.9.791. PMID 8889548.
- Shore EM, Xu M, Shah PB, Janoff HB, Hahn GV, Deardorff MA, Sovinsky L, Spinner NB, Zasloff MA, Wozney JM, Kaplan FS (1998). "The human bone morphogenetic protein 4 (BMP-4) gene: molecular structure and transcriptional regulation". Calcif. Tissue Int. 63 (3): 221–9. doi:10.1007/s002239900518. PMID 9701626.
- Tucker AS, Matthews KL, Sharpe PT (1998). "Transformation of tooth type induced by inhibition of BMP signaling". Science. 282 (5391): 1136–8. Bibcode:1998Sci...282.1136T. doi:10.1126/science.282.5391.1136. PMID 9804553.
- Van den Wijngaard A, Pijpers MA, Joosten PH, Roelofs JM, Van zoelen EJ, Olijve W (1999). "Functional characterization of two promoters in the human bone morphogenetic protein-4 gene". J. Bone Miner. Res. 14 (8): 1432–41. doi:10.1359/jbmr.1999.14.8.1432. PMID 10457277.
- Li W, LoTurco JJ (2000). "Noggin is a negative regulator of neuronal differentiation in developing neocortex". Dev. Neurosci. 22 (1–2): 68–73. doi:10.1159/000017428. PMID 10657699.
- Raatikainen-Ahokas A, Hytönen M, Tenhunen A, Sainio K, Sariola H (2000). "BMP-4 affects the differentiation of metanephric mesenchyme and reveals an early anterior-posterior axis of the embryonic kidney". Dev. Dyn. 217 (2): 146–58. doi:10.1002/(SICI)1097-0177(200002)217:2<146::AID-DVDY2>3.0.CO;2-I. PMID 10706139.
- van den Wijngaard A, Mulder WR, Dijkema R, Boersma CJ, Mosselman S, van Zoelen EJ, Olijve W (2000). "Antiestrogens specifically up-regulate bone morphogenetic protein-4 promoter activity in human osteoblastic cells". Mol. Endocrinol. 14 (5): 623–33. doi:10.1210/me.14.5.623. PMID 10809227.
- Ying Y, Liu XM, Marble A, Lawson KA, Zhao GQ (2000). "Requirement of Bmp8b for the generation of primordial germ cells in the mouse". Mol. Endocrinol. 14 (7): 1053–63. doi:10.1210/mend.14.7.0479. PMID 10894154.
- Nakade O, Takahashi K, Takuma T, Aoki T, Kaku T (2001). "Effect of extracellular calcium on the gene expression of bone morphogenetic protein-2 and -4 of normal human bone cells". J. Bone Miner. Metab. 19 (1): 13–9. doi:10.1007/s007740170055. PMID 11156467.
- Hatta T, Konishi H, Katoh E, Natsume T, Ueno N, Kobayashi Y, Yamazaki T (2001). "Identification of the ligand-binding site of the BMP type IA receptor for BMP-4". Biopolymers. 55 (5): 399–406. doi:10.1002/1097-0282(2000)55:5<399::AID-BIP1014>3.0.CO;2-9. PMID 11241215.
- Aoki H, Fujii M, Imamura T, Yagi K, Takehara K, Kato M, Miyazono K (2001). "Synergistic effects of different bone morphogenetic protein type I receptors on alkaline phosphatase induction". J. Cell Sci. 114 (Pt 8): 1483–9. PMID 11282024.
- Kalinovsky A, Boukhtouche F, Blazeski R, Bornmann C, Suzuki N, Mason CA, Scheiffele P (2011). Polleux F (ed.). "Development of Axon-Target Specificity of Ponto-Cerebellar Afferents". PLOS Biology. 9 (2): e1001013. doi:10.1371/journal.pbio.1001013. PMC 3035609. PMID 21346800.
- Cotsarelis G, Millar SE (July 2001). "Towards a molecular understanding of hair loss and its treatment". Trends Mol Med. 7 (7): 293–301. doi:10.1016/S1471-4914(01)02027-5. PMID 11425637.
- Feijen A, Goumans MJ, van den Eijnden-van Raaij AJ (December 1994). "Expression of activin subunits, activin receptors and follistatin in postimplantation mouse embryos suggests specific developmental functions for different activins". Development. 120 (12): 3621–37. PMID 7821227.
- Graham A, Francis-West P, Brickell P, Lumsden A (December 1994). "The signalling molecule BMP4 mediates apoptosis in the rhombencephalic neural crest". Nature. 372 (6507): 684–6. Bibcode:1994Natur.372..684G. doi:10.1038/372684a0. PMID 7990961.
- Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W (May 2001). "beta-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin". Cell. 105 (4): 533–45. doi:10.1016/S0092-8674(01)00336-1. PMID 11371349.
- Kulessa H, Turk G, Hogan BL (December 2000). "Inhibition of Bmp signaling affects growth and differentiation in the anagen hair follicle". EMBO J. 19 (24): 6664–74. doi:10.1093/emboj/19.24.6664. PMC 305899. PMID 11118201.
- Leong LM, Brickell PM (December 1996). "Bone morphogenic protein-4". Int. J. Biochem. Cell Biol. 28 (12): 1293–6. doi:10.1016/S1357-2725(96)00075-1. PMID 9022288.
- Liem KF, Tremml G, Roelink H, Jessell TM (September 1995). "Dorsal differentiation of neural plate cells induced by BMP-mediated signals from epidermal ectoderm". Cell. 82 (6): 969–79. doi:10.1016/0092-8674(95)90276-7. PMID 7553857.
- Millar SE (February 2002). "Molecular mechanisms regulating hair follicle development". J. Invest. Dermatol. 118 (2): 216–25. doi:10.1046/j.0022-202x.2001.01670.x. PMID 11841536.
- Pourquié O, Fan CM, Coltey M, Hirsinger E, Watanabe Y, Bréant C, Francis-West P, Brickell P, Tessier-Lavigne M, Le Douarin NM (February 1996). "Lateral and axial signals involved in avian somite patterning: a role for BMP4". Cell. 84 (3): 461–71. doi:10.1016/S0092-8674(00)81291-X. PMID 8608600.
- Wang EA, Israel DI, Kelly S, Luxenberg DP (1993). "Bone morphogenetic protein-2 causes commitment and differentiation in C3H10T1/2 and 3T3 cells". Growth Factors. 9 (1): 57–71. doi:10.3109/08977199308991582. PMID 8347351.
- Winnier G, Blessing M, Labosky PA, Hogan BL (September 1995). "Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse". Genes Dev. 9 (17): 2105–16. doi:10.1101/gad.9.17.2105. PMID 7657163.
External links
- BMPedia - the Bone Morphogenetic Protein Wiki
- BMP4 human gene location in the UCSC Genome Browser.
- BMP4 human gene details in the UCSC Genome Browser.