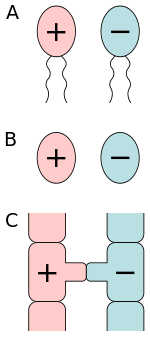
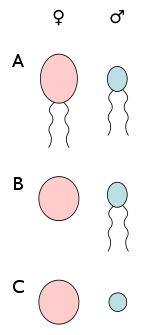
Anisogamy
Anisogamy (also called heterogamy) is the form of sexual reproduction that involves the union or fusion of two gametes, which differ in size and/or form. (The related adjectives are anisogamous and anisogamic.)[1] The smaller gamete is considered to be male (a sperm cell), whereas the larger gamete is regarded as female (typically an egg cell, if non-motile).
There are several types of anisogamy. Both gametes may be flagellated and therefore motile. Alternatively, both of the gametes may be non-flagellated. The latter situation occurs in some algae and plants. In the red alga Polysiphonia, non-motile eggs are fertilized by non-motile sperm. In flowering plants, the gametes are non-motile cells within gametophytes.
The form of anisogamy that occurs in animals, including humans, is oogamy, where a large, non-motile egg (ovum) is fertilized by a small, motile sperm (spermatozoon). The egg is optimized for longevity, whereas the small sperm is optimized for motility and speed. The size and resources of the egg cell allow for the production of pheromones, which attract the swimming sperm cells.[2]
Anisogamy and sexual dimorphism
Anisogamy is a fundamental concept of sexual dimorphism that helps explain phenotypic differences between sexes.[3] In most species a male and female sex exist, both of which are optimized for reproductive potential. Due to their differently sized and shaped gametes, both males and females have developed physiological and behavioral differences that optimize the individual's fecundity.[3] Since most egg laying females typically must bear the offspring and have a more limited reproductive cycle, this typically makes females a limiting factor in the reproductive success rate of males in a species. This process is also true for females selecting males, and assuming that males and females are selecting for different traits in partners, would result in phenotypic differences between the sexes over many generations. This hypothesis, known as the Bateman's Principle, is used to understand the evolutionary pressures put on males and females due to anisogamy.[4] Although this assumption has criticism, it is a generally accepted model for sexual selection within anisogamous species. The selection for different traits depending on sex within the same species is known as sex-specific selection, and accounts for the differing phenotypes found between the sexes of the same species. This sex-specific selection between sexes over time also lead to the development of secondary sex characteristics, which assist males and females in reproductive success.
In most species, both sexes choose mates based on the available phenotypes of potential mates.[4] These phenotypes are species specific, resulting in varying strategies for successful sexual reproduction. For example, large males are sexually selected for in elephant seals for their large size helps the male fight off other males, but small males are sexually selected for in spiders for they can mate with the female more quickly while avoiding sexual cannibalism.[5] However, despite the large range of sexually selected phenotypes, most anisogamous species follow a set of predictable desirable traits and selective behaviors based on general reproductive success models.
Female phenotypes
For internal fertilizers, female investment is high in reproduction since they typically expend more energy throughout a single reproductive event. This can be seen as early as oogenesis, for the female sacrifices gamete number for gamete size to better increase the survival chances of the potential zygote; a process more energetically demanding than spermatogenesis in males.[6] Oogenesis occurs in the ovary, a female specific organ that also produces hormones to prepare other female-specific organs for the changes necessary in the reproductive organs to facilitate egg delivery in external fertilizers, and zygote development in internal fertilizers. The egg cell produced is not only large, but sometimes even immobile, requiring contact with the more mobile sperm to instigate fertilization.[6]
Since this process is very energy-demanding and time-consuming for the female, mate choice is often integrated into the female's behavior.[3] Females will often be very selective of the males they choose to reproduce with, for the phenotype of the male can be indicative of the male's physical health and heritable traits. Females employ mate choice to pressure males into displaying their desirable traits to females through courtship, and if successful, the male gets to reproduce. This encourages males and females of specific species to invest in courtship behaviors as well as traits that can display physical health to a potential mate. This process, known as sexual selection,[3] results in the development of traits to ease reproductive success rather than individual survival, such as the inflated size of a termite queen. It is also important for females to select against potential mates that may have a sexually transmitted infection, for the disease could not only hurt the female's reproductive ability, but also damage the resulting offspring.[7]
Although not uncommon in males, females are more associated with parental care.[8] Since females are on a more limited reproductive schedule than males, a female often invests more in protecting the offspring to sexual maturity than the male. Like mate choice, the level of parental care varies greatly between species, and is often dependent on the number of offspring produced per sexual encounter.[8]
In most species such as Drosophila melanogaster, females can utilize sperm storage,[9] a process by which the female can store excess sperm from a mate, and fertilize her eggs long after the reproductive event if mating opportunities drop or quality of mates decreases. By being able to save sperm from more desirable mates, the female gains more control over its own reproductive success, thus allowing for the female to be more selective of males as well as making the timing of fertilization potentially more frequent if males are scarce.[9]
Male phenotypes
For males of all species, the sperm cells they produce are optimized for ensuring fertilization of the female egg. These sperm cells are created through spermatogenesis, a form of gametogenesis that focuses on developing the most possible gametes per sexual encounter.[6] Spermatogenesis occurs in the testes, a male specific organ that is also produces hormones that trigger the development of secondary sex characteristics. Since the male's gametes are energetically cheap and abundant in every ejaculation, a male can greatly increase his sexual success by mating far more frequently than the female.[6] Sperm, unlike egg cells, are also mobile, allowing for the sperm to swim towards the egg through the female's sexual organs. Sperm competition is also a major factor in the development of sperm cells. Only one sperm can fertilize an egg, and since females can potentially reproduce with more than one male before fertilization occurs, producing sperm cells that are faster, more abundant, and more viable than that produced by other males can give a male reproductive advantage.[6]
Since females are often the limiting factor in a species reproductive success, males are often expected by the females to search and compete for the female, known as intraspecific competition.[4] This can be seen in organisms such as bean beetles, as the male that searches for females more frequently is often more successful at finding mates and reproducing. In species undergoing this form of selection, a fit male would be one that is fast, has more refined sensory organs, and spatial awareness.[4]
Some secondary sex characteristics are not only meant for attracting mates, but also for competing with other males for copulation opportunities. Some structures, such as antlers in deer, can provide benefits to the male's reproductive success by providing a weapon to prevent rival males from achieving reproductive success.[7] However, other structures such as the large colorful tail feathers found in male peacocks, are a result of Fisherian Runaway as well as several more species specific factors. Due to females selecting for specific traits in males, over time, these traits are exaggerated to the point where they could hinder the male's survivability.[7] However, since these traits greatly benefit sexual selection, their usefulness in providing more mating opportunities overrides the possibility that the trait could lead to a shortening of its lifespan through predation or starvation. These desirable traits extend beyond physical body parts, and often extend into courtship behavior and nuptial gifts as well.
Although some behaviors in males are meant to work within the parameters of cryptic female choice, some male traits work against it. Strong enough males, in some cases, can force themselves upon a female, forcing fertilization and overriding female choice.[10] Since this can often be dangerous for the female, an Evolutionary Arms Race between the sexes is often an outcome.
Evolution
Anisogamy is the phenomenon of fertilization of large gametes (egg cells, ova) by (or with) small gametes (sperm cells: spermatozoa or spermatia). Gamete size difference is the fundamental difference between females and males. Anisogamy first evolved in multicellular haploid species after the differentiation of different mating types had already been established.[11] However, in Ascomycetes, anisogamy evolved from isogamy before mating types.[12]
The three main theories for the evolution of anisogamy are gamete competition, gamete limitation, and intracellular conflicts, but the last of these three is not well supported by current evidence.[13] Both gamete competition and gamete limitation assume that anisogamy originated through disruptive selection acting on an ancestral isogamous population with external fertilization, due to a trade-off between larger gamete number and gamete size (which in turn affects zygote survival), because the total resource one individual can invest in reproduction is assumed to be fixed.[14]
The first formal, mathematical theory proposed to explain the evolution of anisogamy was based on gamete limitation:[15] this model assumed that natural selection would lead to gamete sizes that result in the largest population-wide number of successful fertilizations.[15][16][17] If it is assumed that a certain amount of resources provided by the gametes are needed for the survival of the resulting zygote, and that there is a trade-off between the size and number of gametes, then this optimum was shown to be one where both small (male) and large (female) gametes are produced. However, these early models assume that natural selection acts mainly at the population level, something that is today known to be a very problematic assumption.[18]
The first mathematical model to explain the evolution of anisogamy via individual level selection, and one that became widely accepted was the theory of gamete or sperm competition.[19][20][21] Here, selection happens at the individual level: those individuals that produce more (but smaller) gametes also gain a larger proportion of fertilizations simply because they produce a larger number of gametes that 'seek out' those of the larger type. However, because zygotes formed from larger gametes have better survival prospects, this process can again lead to the divergence of gametes sizes into large and small (female and male) gametes. The end result is one where it seems that the numerous, small gametes compete for the large gametes that are tasked with providing maximal resources for the offspring.
Some recent theoretical work has challenged the gamete competition theory, by showing that gamete limitation by itself can lead to the divergence of gamete sizes even under selection at the individual level.[22][23][24] While this is possible, it has also been shown that gamete competition and gamete limitation are the ends of a continuum of selective pressures, and they can act separately or together depending on the conditions.[25] These selection pressures also act in the same direction (to increase gamete numbers at the expense of size) and at the same level (individual selection). Theory also suggests that gamete limitation could only have been the dominant force of selection for the evolutionary origin of the sexes under quite limited circumstances, and the presence on average of just one competitor can makes the 'selfish' evolutionary force of gamete competition stronger than the 'cooperative' force of gamete limitation even if gamete limitation is very acute (approaching 100% of eggs remaining unfertilized).[26]
There is then a relatively sound theory base for understanding this fundamental transition from isogamy to anisogamy in the evolution of reproduction, which is predicted to be associated with the transition to multicellularity. In fact, Hanschen et al. (2018) demonstrate that anisogamy evolved from isogamous multicellular ancestors and that anisogamy would subsequently drive secondary sexual dimorphism.[11] Some comparative empirical evidence for the gamete competition theories exists,[13][27][28] although it is difficult to use this evidence to fully tease apart the competition and limitation theories because their testable predictions are similar.[14] It has also been claimed that some of the organisms used in such comparative studies do not fit the theoretical assumptions well.[29]
A valuable model system to the study of the evolution of anisogamy is the volvocine algae, which group of chlorophytes is quite unique for its extant species exhibit a diversity of mating systems (isogamy and anisogamy) in addition to its extremes in both unicellularity and multicellularity with a diversity of forms in species of intermediate ranges of sizes.[30] Marine algae have been closely studied to understand the trajectories of such diversified reproductive systems,[31] evolution of sex and mating types,[32] as well as the adaptiveness and stability of anisogamy.[33][31][11]
See also
- Bateman's principle
- Evolution of sex
- Gamete
- Isogamy
- Meiosis
- Sex
References
- "Anisogamy".
- Dusenbery, David B. (2009). "Chapter 20". Living at Micro Scale. Cambridge, Mass: Harvard University Press. ISBN 978-0-674-03116-6.
- De Lisle, SP; Rowe, L (1803). "Independent evolution of the sexes promotes amphibian diversification". Proceedings of the Royal Society B: Biological Sciences. 282: 20142213. doi:10.1098/rspb.2014.2213.
- Fritzsche, K; Arnqvis, G (2013). "HOMAGE TO BATEMAN: SEX ROLES PREDICT SEX DIFFERENCES IN SEXUAL SELECTION". Evolution. 67 (7): 1926–1936. doi:10.1111/evo.12086. PMID 23815650.
- Anne Danielson-François, Chueh Hou, Nina Cole, I-Min Tso, Scramble competition for moulting females as a driving force for extreme male dwarfism in spiders, Animal Behaviour, Volume 84, Issue 4, October 2012, Pages 937-945, ISSN 0003-3472
- Keyne Monro, Dustin J. Marshall Unravelling anisogamy: egg size and ejaculate size mediate selection on morphology in free-swimming sperm
- Davies, N. B., Krebs, J. R., & West, S. A. (2012). An introduction to behavioural ecology. Oxford: Wiley-Blackwell.
- Formhage, L., & Jennions, M. (n.d.). Coevolution of parental investment and sexually selected
- Nakadera, Y.; Koene, J. M. (2013). "Reproductive strategies in hermaphroditic gastropods: Conceptual and empirical approaches". Canadian Journal of Zoology. 91 (6): 367–381. doi:10.1139/cjz-2012-0272.
- Friesen, C. R.; Uhrig, E. J.; Mason, R. T.; Brennan, P. L. R. (2016). "Female behaviour and the interaction of male and female genital traits mediate sperm transfer during mating". Journal of Evolutionary Biology. 29 (5): 952–964. doi:10.1111/jeb.12836. PMID 26809830.
- Hanschen, Erik R.; Herron, Matthew D.; Wiens, John J.; Nozaki, Hisayoshi; Michod, Richard E. (2018). "Multicellularity Drives the Evolution of Sexual Traits" (PDF). The American Naturalist. 192 (3): E93–E105. doi:10.1086/698301. ISSN 0003-0147. PMID 30125231.
- Beukeboom, L. & Perrin, N. (2014). The Evolution of Sex Determination. Oxford University Press, p. 25 . Online resources, .
- Lessells C.M., Snook R.R., Hosken D.J. 2009 The evolutionary origin and maintenance of sperm: selection for a small, motile gamete mating type. In Sperm biology: An evolutionary perspective (eds. Birkhead T.R., Hosken D.J., Pitnick S.), pp. 43-67. London, Academic press.
- Lehtonen, J.; Parker, G.A. (2014). "Gamete competition, gamete limitation, and the evolution of the two sexes". Molecular Human Reproduction. 20 (12): 1161–1168. doi:10.1093/molehr/gau068. PMID 25323972.
- Kalmus, H (1932). "Über den Erhaltungswert der phänotypischen (morphologischen) Anisogamie und die Entstehung der ersten Geschlechtsunterschiede". Biologisches Zentralblatt. 52: 716–736.
- Scudo, F.M. (1967). "Adaptive value of sexual dimorphism - I, anisogamy". Evolution. 21 (2): 285–291. doi:10.2307/2406676. JSTOR 2406676. PMID 28556138.
- Dusenbery, D.B. (2000). "Selection for high gamete encounter rates explains the success of male and female mating types". Journal of Theoretical Biology. 202 (1): 1–10. CiteSeerX 10.1.1.408.1475. doi:10.1006/jtbi.1999.1017. PMID 10623494.
- Williams G.C., 1966, "Adaptation and natural selection: a critique of some current evolutionary thoughts". Princeton, NJ.
- Parker, G.A.; Baker, R.R.; Smith, V.G.F. (1972). "The origin and evolution of gamete dimorphism and the male-female phenomenon". Journal of Theoretical Biology. 36 (3): 529–553. doi:10.1016/0022-5193(72)90007-0.
- Parker, G.A. (1978). "Selection on non-random fusion of gametes during evolution of anisogamy". Journal of Theoretical Biology. 73 (1): 1–28. doi:10.1016/0022-5193(78)90177-7. PMID 567721.
- Parker, G.A. (1982). "Why are there so many tiny sperm? Sperm competition and the maintenance of two sexes". Journal of Theoretical Biology. 96 (2): 281–294. doi:10.1016/0022-5193(82)90225-9. PMID 7121030.
- Cox, P.A.; Sethian, J.A. (1985). "Gamete motion, search, and the evolution of anisogamy, oogamy, and chemotaxis". American Naturalist. 125 (1): 74–101. doi:10.1086/284329.
- Iyer, P.; Roughgarden, J. (2008). "Gametic conflict versus contact in the evolution of anisogamy". Theoretical Population Biology. 73 (4): 461–472. doi:10.1016/j.tpb.2008.02.002. PMID 18485981.
- Yang, J.-N. (2010). "Cooperation and the evolution of anisogamy". Journal of Theoretical Biology. 264 (1): 24–36. doi:10.1016/j.jtbi.2010.01.019. PMID 20097207.
- Lehtonen, J.; Kokko, H. (2011). "Two roads to two sexes: unifying gamete competition and gamete limitation in a single model of anisogamy evolution". Behav Ecol Sociobiol. 65 (3): 445–459. doi:10.1007/s00265-010-1116-8.
- Parker, G.A.; Lehtonen, J. (2014). "Gamete evolution and sperm numbers: sperm competition versus sperm limitation". Proceedings of the Royal Society B: Biological Sciences. 281 (1791): 1791. doi:10.1098/rspb.2014.0836. PMC 4132674. PMID 25100694.
- Knowlton, N (1974). "A note on the evolution of gamete dimorphism". Journal of Theoretical Biology. 46 (1): 283–285. doi:10.1016/0022-5193(74)90153-2.
- Parker G.A., 2011, "The origin and maintenance of two sexes (anisogamy), and their gamete sizes by gamete competition". The evolution of anisogamy (eds. Togashi T., Cox P.A.), pp. 17-74. Cambridge, Cambridge University Press.
- Randerson, J.P.; Hurst, L.D. (2001). "The uncertain evolution of the sexes". Trends in Ecology & Evolution. 16 (10): 571–579. doi:10.1016/s0169-5347(01)02270-4.
- Ferris, Patrick; Olson, Bradley J.S.C.; De Hoff, Peter L.; Douglass, Stephen; Diaz-Cano, David Casero; Prochnik, Simon; Geng, Sa; Rai, Rhitu; Grimwood, Jane (2010-04-16). "Evolution of an Expanded Sex Determining Locus in Volvox". Science. 328 (5976): 351–354. doi:10.1126/science.1186222. ISSN 0036-8075. PMC 2880461. PMID 20395508.
- Togashi, Tatsuya; Bartelt, John L.; Yoshimura, Jin; Tainaka, Kei-ichi; Cox, Paul Alan (2012). "Evolutionary trajectories explain the diversified evolution of isogamy and anisogamy in marine green algae". Proceedings of the National Academy of Sciences of the United States of America. 109 (34): 13692–13697. doi:10.1073/pnas.1203495109. ISSN 1091-6490. PMC 3427103. PMID 22869736.
- Herron, Matthew D. (2016). "Origins of multicellular complexity: Volvox and the volvocine algae". Molecular Ecology. 25 (6): 1213–1223. doi:10.1111/mec.13551. ISSN 1365-294X. PMC 5765864. PMID 26822195.
- Cox, P. A.; Sethian, J. A. (1984). "Search, encounter rates, and the evolution of anisogamy". Proceedings of the National Academy of Sciences of the United States of America. 81 (19): 6078–6079. doi:10.1073/pnas.81.19.6078. ISSN 0027-8424. PMC 391862. PMID 6592603.