2-Naphthalenethiol
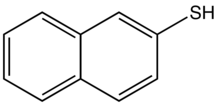
2-Naphthalenethiol is an organosulfur compound with the formula C10H7SH. It is a white solid. It is one of two monothiols of naphthalene, the other being 1-naphthalenethiol.
 | |
| Names | |
|---|---|
| IUPAC name
Naphthalene-2-thiol | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.001.893 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H8S | |
| Molar mass | 160.23 g·mol−1 |
| Appearance | White solid |
| Melting point | 80–81 °C (176–178 °F; 353–354 K) |
| Boiling point | 92–94 °C (198–201 °F; 365–367 K) (at 0.4 mmHg) |
| Hazards | |
| GHS pictograms |  |
| GHS Signal word | Warning |
GHS hazard statements |
H302 |
| P264, P270, P301+312, P330, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Synthesis and reactions
2-Naphthalenethiol is prepared from 2-naphthol by the Newman–Kwart rearrangement using the thiocarbamate.[1] It undergoes lithiation at the 1 and 3-position.[2][3]
It can be used as a flavouring agent.[4]
gollark: I am NOT being paid by pizza companies to subliminally advertise tasty pizza, available now, visit https://pizza.pizza, by the way.
gollark: Oh, now you assume that I can just "edit images" now.
gollark: How could you mistake tilings of the hyperbolic plane for pizza?
gollark: Why does EVERYONE keep assuming it's pizzæ?
gollark: It is currently free of bees.
References
- Melvin S. Newman; Frederick W. Hetzel (1971). "Thiophenols from Phenols: 2-Naphthalenethiol". Org. Synth. 51: 139. doi:10.15227/orgsyn.051.0139.
- Block, E.; Eswarakrishnan, V.; Gernon, M.; Ofori-Okai, G.; Saha, C.; Tang, K.; Zubieta, J. (1989). "o-Lithiothiophenol Equivalents. Generation, Reactions and Applications in Synthesis of Hindered Thiolate Ligands". J. Am. Chem. Soc. 111: 658–665. doi:10.1021/ja00184a039.
- Still, Ian WJ; Natividad-Preyra, Rosanne; Toste, F Dean (1999). "A versatile synthetic route to 1,5-dithiocins from o-mercapto aromatic aldehydes". Canadian Journal of Chemistry. 77: 113–121. doi:10.1139/v98-230.
- WHO, World Health. "Evaluations of the Joint FAO/WHO Expert Committee on Food Additives (JECFA) Feedback Print preview Link to this page 2-NAPHTHALENETHIOL". apps.who.int. Retrieved 15 January 2018.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.