Tiopronin
Tiopronin (trade name Thiola) is a prescription thiol drug used to control the rate of cystine precipitation and excretion in the disease cystinuria.[1][2] Due to the rarity of the disorder, tiopronin falls under the classification of an orphan drug. It is somewhat similar to penicillamine in both chemistry and pharmacology.
 | |
| Names | |
|---|---|
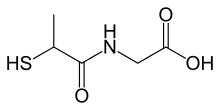
| IUPAC name
2-(2-sulfanylpropanoylamino)acetic acid | |
| Other names
2-mercaptopropionylglycine Acadione Thiola | |
| Identifiers | |
3D model (JSmol) |
|
| 1859822 | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.016.163 |
| EC Number |
|
| KEGG | |
| MeSH | Tiopronin |
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C5H9NO3S | |
| Molar mass | 163.19 g·mol−1 |
| Appearance | White, opaque crystals |
| Melting point | 93 to 98 °C (199 to 208 °F; 366 to 371 K) |
| log P | −0.674 |
| Acidity (pKa) | 3.356 |
| Basicity (pKb) | 10.641 |
| Pharmacology | |
| G04BX16 (WHO) | |
| |
| Legal status |
|
| Hazards | |
| GHS pictograms |  |
| GHS Signal word | Warning |
GHS hazard statements |
H302 |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
1,300 mg kg−1 (oral, rat) |
| Related compounds | |
Related alkanoic acids |
|
Related compounds |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Uses
Tiopronin is used primarily for cystinuria and is well known in the cystinuric community. Depending on the severity of a person's cystinuria, tiopronin may be taken for life, possibly starting in early childhood. The drug works by reacting with urinary cysteine to form a more soluble, disulfide linked, tiopronin-cysteine complex.[3]
It may also be used for Wilson's disease (an overload of copper in the body), and has also been investigated for the treatment of arthritis,[4][5] though tiopronin is not an anti-inflammatory.
Tiopronin is also sometimes used as a stabilizing agent for metal nanoparticles. The thiol group binds to the nanoparticles, preventing coagulation.[6]
Side effects
Tiopronin may present a variety of side effects, which are broadly similar to those of D-penicillamine and other compounds containing active sulfhydryl groups.[7] Its pharmacokinetics have been studied.[3]
Society and culture
In the U.S., the drug was marketed by Mission Pharmacal at $1.50 per pill, but in 2014 the rights were bought by Retrophin, owned by Martin Shkreli, and the price increased to $30 per pill for a 100mg capsule.[8][9]
In 2016 Imprimis Pharmaceuticals introduced a lower cost version marketed as a compounded drug.[10]
References
- Lindell, Å.; Denneberg, T.; Hellgren, E.; Jeppsson, J. -O.; Tiselius, H. -G. (1995). "Clinical course and cystine stone formation during tiopronin treatment". Urological Research. 23 (2): 111–117. doi:10.1007/BF00307941. PMID 7676533.
- Coe, Fredric L.; Parks, Joan H.; Asplin, John R. (15 October 1992). "The Pathogenesis and Treatment of Kidney Stones". New England Journal of Medicine. 327 (16): 1141–1152. doi:10.1056/NEJM199210153271607. PMID 1528210.
- Carlsson, M. S.; Denneberg, T.; Emanuelsson, B.-M.; Kågedal, B.; Lindgren, S. (August 1993). "Pharmacokinetics of oral tiopronin". European Journal of Clinical Pharmacology. 45 (1): 79–84. doi:10.1007/BF00315354. PMID 8405034.
- Delecoeuillerie, G (30 April 1989). "[Tolerability and therapeutic maintenance of tiopronin, new basic treatment of rheumatoid arthritis. Apropos of long-term follow-up of 268 cases]". Revue du Rhumatisme et des Maladies Osteo-articulaires. 56 (5 Pt 2): 38–42. PMID 2740804.
- Pasero, Giampiero; Pellegrini, Pietro; Ambanelli, Umberto; Ciompi, Maria Laura; Colamussi, Vincenzo; Ferraccioli, Gianfranco; Barbieri, Paola; Mazzoni, Maria Rosa; Menegale, Germano; Trippi, Donatella (August 1982). "Controlled multicenter trial of tiopronin and D-penicillamine for rheumatoid arthritis". Arthritis & Rheumatism. 25 (8): 923–929. doi:10.1002/art.1780250803.
- Jennifer A. Dahl; Bettye L. S. Maddux & James E. Hutchison (2007). "Toward Greener Nanosynthesis". Chemical Reviews. 107 (6): 2228–2269. CiteSeerX 10.1.1.454.2724. doi:10.1021/cr050943k. PMID 17564480.
- Jaffe, Israeli A. (March 1986). "Adverse effects profile of sulfhydryl compounds in man". The American Journal of Medicine. 80 (3): 471–476. doi:10.1016/0002-9343(86)90722-9. PMID 2937293.
- Lowe, Derek (11 September 2014). "The Most Unconscionable Drug Price Hike I Have Yet Seen". In the Pipeline.
- Carroll, John (September 20, 2015). "Why would Martin Shkreli hike an old drug price by 5000%? Only a 'moron' would ask". FierceBiotech.
- Elvidge, Suzanne (September 29, 2016). "Imprimis shuts down Texas plant, axes 8% of jobs". BioPharma Dive.