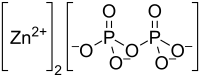
Zinc pyrophosphate
Zinc pyrophosphate (Zn2P2O7) is an ionic inorganic chemical compound composed of Zn2+ cations and pyrophosphate anions. It is useful in gravimetric analysis of zinc. Zinc pyrophosphate is obtained by precipitating zinc as a phosphate, then heating over 1123 K.
 | |
| Names | |
|---|---|
| Other names
Dizinc diphosphate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.028.367 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| Zn2P2O7 | |
| Molar mass | 304.72 g/mol |
| Appearance | White crystalline powder |
| Density | 3.75 g/cm3 |
| Insoluble | |
| Solubility | Soluble in dilute acids |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
References
- Lide, David R. (1998), Handbook of Chemistry and Physics (87 ed.), Boca Raton, Florida: CRC Press, pp. 4–96, ISBN 0-8493-0594-2
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.