Zinc molybdate
Zinc molybdate is an inorganic compound with the formula ZnMoO4. It is used as a white pigment, which that is also a corrosion inhibitor. A related pigment is sodium zinc molybdate, Na2Zn(MoO4)2.[3] The material has also been investigated as an electrode material.[4]
 | |
| Identifiers | |
|---|---|
| ECHA InfoCard | 100.033.965 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| Properties | |
| ZnMoO4 | |
| Molar mass | 225.33 g/mol |
| Appearance | white tetragonal crystals |
| Density | 4.32 g/cm3[2] |
| Melting point | 900 °C (1,650 °F; 1,170 K) |
| insoluble | |
| Structure | |
| tetragonal | |
| Hazards | |
EU classification (DSD) (outdated) |
not listed |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
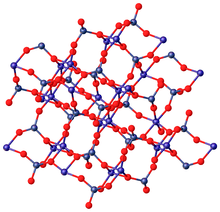
In terms of its structure, the Mo(VI) centers are tetrahedral and the Zn(II) centers are octahedral.[2]
Safety
The LD50 (oral, rats) is 11,500 mg/kg.[3] While highly soluble molybdates like e.g. sodium molybdate are toxic in higher doses, zinc molybdate is essentially non-toxic because of its insolubility in water. Molybdates possess a lower toxicity than chromates or lead salts and are therefore seen as an alternative to these salts for corrosion inhibition.
gollark: I bet 5 RPi4s would outperform that.
gollark: Are those *original* Raspberry Pies?
gollark: Actually, I own this channel, by divine right.
gollark: Even if you could make a black hole here, it would hardly be much use, you can't look inside it or something.
gollark: Sadly, galaxies are quite big and communications can (with current technology) only go at the speed of light.
References
- Lide, David R. (1998), Handbook of Chemistry and Physics (87 ed.), Boca Raton, FL: CRC Press, pp. 4–95, ISBN 978-0-8493-0594-8
- Ait Ahsaine, H.; Zbair, M.; Ezahri, M.; Benlhachemi, A.; Arab, M.; Bakiz, B.; Guinneton, F.; Gavarri, J. R. (2015). "Rietveld Refinements, Impedance Spectroscopy and Phase Transition of the Polycrystalline ZnMoO4 Ceramics". Ceramics International. 41 (10): 15193–15201. doi:10.1016/j.ceramint.2015.08.094.CS1 maint: multiple names: authors list (link)
- G. Etzrodt (2012). "Pigments, Inorganic 5. Anticorrosive Pigments". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.n20_n04.
- Hu, Xianluo; Zhang, Wei; Liu, Xiaoxiao; Mei, Yueni; Huang, Yunhui (2015). "Nanostructured Mo-based electrode materials for electrochemical Energy Storage". Chemical Society Reviews. 44 (8): 2376–404. doi:10.1039/C4CS00350K. PMID 25688809.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
