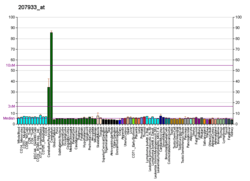
ZP2
Zona pellucida sperm-binding protein 2 is a protein that in humans is encoded by the ZP2 gene.[5][6]
Function
The zona pellucida is an extracellular matrix that surrounds the oocyte and early embryo. It is composed primarily of three (mouse) or four (human) glycoproteins (ZP1-4) with various functions during fertilization and preimplantation development. The protein encoded by this gene is a structural component of the zona pellucida and functions in secondary binding and penetration of acrosome-reacted spermatozoa. The nascent protein contains a N-terminal signal peptide sequence, a conserved ZP domain, a consensus furin cleavage site, and a C-terminal transmembrane domain. It is hypothesized that furin cleavage results in release of the mature protein from the plasma membrane for subsequent incorporation into the zona pellucida matrix. However, the requirement for furin cleavage in this process remains controversial based on mouse studies.[6]
The sperm-binding domain on the ZP2 protein is necessary in both humans and mice for oocyte-sperm recognition and penetration of the zona pellucida. It is also responsible for the primary block to polyspermy in mammals. The oocyte has cortical granules peripherally located under the cortex that contain a proteolytic protein called ovastacin. After the sperm binds to ZP2, the cortical granules are exocytosed releasing ovastacin into the perivitelline space. Ovastacin cleaves ZP2 at the N terminus, preventing more sperm from binding and penetrating the oocyte, thus hardening the zona pellucida. Ovastacin is only found in oocytes, and is part of the astacin family of metalloendoproteases. Female mice engineered without ovastacin showed that ZP2 was not cleaved after fertilization.[7][8]
3D structure
The crystal structure of the sperm-binding domain of ZP2 at 0.95 Å resolution (PDB: 5II6)[9] showed that is shares the same ZP-N fold first identified in structures of ZP3 (PDB: 3D4C, 3D4G, 3EF7, 3NK3, 3NK4).[10][11] This provided experimental evidence for the suggestion that the N-terminal region of ZP2 consists of three ZP-N repeats [10][12] and revealed that - despite insignificant sequence identity - ZP2 is structurally similar to VERL, the vitelline envelope receptor for egg lysin of the mollusk abalone (PDB: 5II4, 5II5, 5MR2, 5IIC, 5IIA, 5IIB, 5MR3). This established a link between invertebrate and vertebrate fertilization by suggesting that, despite being separated by 600 million years of evolution, mollusk and human use a common protein fold to interact with sperm.[9]
References
- ENSG00000103310 GRCh38: Ensembl release 89: ENSG00000284588, ENSG00000103310 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000030911 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Liang LF, Dean J (Apr 1993). "Conservation of mammalian secondary sperm receptor genes enables the promoter of the human gene to function in mouse oocytes". Dev Biol. 156 (2): 399–408. doi:10.1006/dbio.1993.1087. PMID 8385033.
- "Entrez Gene: ZP2 zona pellucida glycoprotein 2 (sperm receptor)".
- Burkart AD, Xiong B, Baibakov B, Jiménez-Movilla M, Dean J (2012). "Ovastacin, a cortical granule protease, cleaves ZP2 in the zona pellucida to prevent polyspermy". J Cell Biol. 197 (1): 37–44. doi:10.1083/jcb.201112094. PMC 3317803. PMID 22472438.
- Avella MA, Baibakov B, Dean J (2014). "A single domain of the ZP2 zona pellucida protein mediates gamete recognition in mice and humans". J Cell Biol. 205 (6): 801–809. doi:10.1083/jcb.201404025. PMC 4068139. PMID 24934154.
- Raj I, Sadat Al Hosseini H, Dioguardi E, Nishimura K, Han L, Villa A, de Sanctis D, Jovine L (2017). "Structural Basis of Egg Coat-Sperm Recognition at Fertilization". Cell. 169 (7): 1315–1326. doi:10.1016/j.cell.2017.05.033. PMC 5480393. PMID 28622512. PDB: 5II6, 5II4, 5II5, 5MR2, 5IIC, 5IIA, 5IIB, 5MR3
- Monné M, Han L, Schwend T, Burendahl S, Jovine L (2008). "Crystal structure of the ZP-N domain of ZP3 reveals the core fold of animal egg coats". Nature. 456 (7222): 653–7. Bibcode:2008Natur.456..653M. doi:10.1038/nature07599. PMID 19052627. PDB: 3D4C, 3D4G, 3EF7
- Han L, Monné M, Okumura H, Schwend T, Cherry AL, Flot D, Matsuda T, Jovine L (2010). "Insights into egg coat assembly and egg-sperm interaction from the X-ray structure of full-length ZP3". Cell. 143 (3): 404–15. doi:10.1016/j.cell.2010.09.041. PMID 20970175. PDB: 3NK3, 3NK4
- Callebaut I, Mornon JP, Monget P (2007). "Isolated ZP-N domains constitute the N-terminal extensions of Zona Pellucida proteins". Bioinformatics. 23 (15): 1871–1874. doi:10.1093/bioinformatics/btm265. PMID 17510169.
Further reading
- Rankin T, Dean J (2000). "The zona pellucida: using molecular genetics to study the mammalian egg coat". Rev. Reprod. 5 (2): 114–121. doi:10.1530/ror.0.0050114. PMID 10864856.
- Mori T, Guo MW, Sato E, Baba T, Takasaki S, Mori E (2000). "Molecular and immunological approaches to mammalian fertilization". J. Reprod. Immunol. 47 (2): 139–158. doi:10.1016/S0165-0378(00)00055-3. PMID 10924747.
- Loftus BJ, Kim UJ, Sneddon VP, Kalush F, Brandon R, Fuhrmann J, Mason T, Crosby ML, Barnstead M, Cronin L, Deslattes Mays A, Cao Y, Xu RX, Kang HL, Mitchell S, Eichler EE, Harris PC, Venter JC, Adams MD (1999). "Genome duplications and other features in 12 Mb of DNA sequence from human chromosome 16p and 16q". Genomics. 60 (3): 295–308. doi:10.1006/geno.1999.5927. PMID 10493829.
- Tsubamoto H, Hasegawa A, Nakata Y, Naito S, Yamasaki N, Koyama K (1999). "Expression of recombinant human zona pellucida protein 2 and its binding capacity to spermatozoa". Biol. Reprod. 61 (6): 1649–1654. doi:10.1095/biolreprod61.6.1649. PMID 10570015.
- Howes E, Pascall JC, Engel W, Jones R (2002). "Interactions between mouse ZP2 glycoprotein and proacrosin; a mechanism for secondary binding of sperm to the zona pellucida during fertilization". J. Cell Sci. 114 (Pt 22): 4127–36. PMID 11739644.
- Tanii I, Oh-oka T, Yoshinaga K, Toshimori K (2002). "A mouse acrosomal cortical matrix protein, MC41, has ZP2-binding activity and forms a complex with a 75-kDa serine protease". Dev. Biol. 238 (2): 332–341. doi:10.1006/dbio.2001.0380. PMID 11784014.
- Kiefer SM, Saling P (2002). "Proteolytic processing of human zona pellucida proteins". Biol. Reprod. 66 (2): 407–414. doi:10.1095/biolreprod66.2.407. PMID 11804956.
- Qi H, Williams Z, Wassarman PM (2002). "Secretion and assembly of zona pellucida glycoproteins by growing mouse oocytes microinjected with epitope-tagged cDNAs for mZP2 and mZP3". Mol. Biol. Cell. 13 (2): 530–541. doi:10.1091/mbc.01-09-0440. PMC 65647. PMID 11854410.
- Zhao M, Gold L, Ginsberg AM, Liang LF, Dean J (2002). "Conserved furin cleavage site not essential for secretion and integration of ZP3 into the extracellular egg coat of transgenic mice". Mol. Cell. Biol. 22 (9): 3111–3120. doi:10.1128/MCB.22.9.3111-3120.2002. PMC 133755. PMID 11940668.
- Rankin TL, Coleman JS, Epifano O, Hoodbhoy T, Turner SG, Castle PE, Lee E, Gore-Langton R, Dean J (2003). "Fertility and taxon-specific sperm binding persist after replacement of mouse sperm receptors with human homologs". Dev. Cell. 5 (1): 33–43. doi:10.1016/S1534-5807(03)00195-3. PMID 12852850.
- Hinsch E, Groeger S, Oehninger S, Hinsch KD (2003). "Localization and functional importance of a conserved zona pellucida 2 protein domain in the human and bovine ovary using monoclonal anti-ZP2 peptide antibodies". Theriogenology. 60 (7): 1331–1344. doi:10.1016/S0093-691X(03)00169-9. PMID 14511786.
- Furlong LI, Harris JD, Vazquez-Levin MH (2006). "Binding of recombinant human proacrosin/acrosin to zona pellucida (ZP) glycoproteins. I. Studies with recombinant human ZPA, ZPB, and ZPC". Fertil. Steril. 83 (6): 1780–1790. doi:10.1016/j.fertnstert.2004.12.042. PMID 15950651.
- Caballero-Campo P, Chirinos M, Fan XJ, González-González ME, Galicia-Chavarría M, Larrea F, Gerton GL (2006). "Biological effects of recombinant human zona pellucida proteins on sperm function". Biol. Reprod. 74 (4): 760–768. doi:10.1095/biolreprod.105.047522. PMID 16407501.