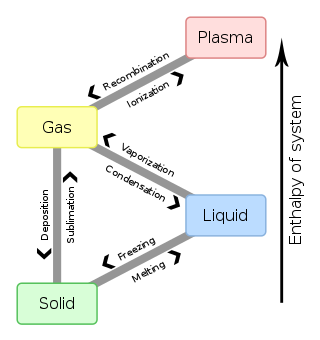
Supercooling
Supercooling,[1] also known as undercooling,[2] is the process of lowering the temperature of a liquid or a gas below its freezing point without it becoming a solid. It achieves this in the absence of a seed crystal or nucleus around which a crystal structure can form. The supercooling of water can be achieved without any special techniques other than chemical demineralization, down to −48.3 °C (−55 °F). Droplets of supercooled water often exist in stratus and cumulus clouds. An aircraft flying through such a cloud sees an abrupt crystallization of these droplets, which can result in the formation of ice on the aircraft's wings or blockage of its instruments and probes.
Animals utilize supercooling to survive in extreme temperatures, as a last resort only. There are many techniques that aid in maintaining a liquid state, such as the production of antifreeze proteins, which bind to ice crystals to prevent water molecules from binding and spreading the growth of ice.[3] The winter flounder is one such fish that utilizes these proteins to survive in its frigid environment. In plants, cellular barriers such as lignin, suberin and the cuticle inhibit ice nucleators and force water into the supercooled tissue.
One commercial application of supercooling is in refrigeration. Freezers can cool drinks to a supercooled level so that when they are opened, they form a slush. Supercooling was also successfully applied to organ preservation at Massachusetts General Hospital/Harvard Medical School. Livers that were later transplanted into recipient animals were preserved by supercooling for up to 96 hours (4 days), quadrupling the limits of what could be achieved by conventional liver preservation methods.
Explanation
A liquid crossing its standard freezing point will crystalize in the presence of a seed crystal or nucleus around which a crystal structure can form creating a solid. Lacking any such nuclei, the liquid phase can be maintained all the way down to the temperature at which crystal homogeneous nucleation occurs.
Homogeneous nucleation can occur above the glass transition temperature, but if homogeneous nucleation has not occurred above that temperature, an amorphous (non-crystalline) solid will form.
Water normally freezes at 273.15 K (0 °C or 32 °F), but it can be "supercooled" at standard pressure down to its crystal homogeneous nucleation at almost 224.8 K (−48.3 °C/−55 °F).[4][5] The process of supercooling requires that water be pure and free of nucleation sites, which can be achieved by processes like reverse osmosis or chemical demineralization, but the cooling itself does not require any specialised technique. If water is cooled at a rate on the order of 106 K/s, the crystal nucleation can be avoided and water becomes a glass—that is, an amorphous (non-crystalline) solid. Its glass transition temperature is much colder and harder to determine, but studies estimate it at about 136 K (−137 °C/−215 °F).[6] Glassy water can be heated up to approximately 150 K (−123 °C/−189.4 °F) without nucleation occurring.[5] In the range of temperatures between 231 K (−42 °C/−43.6 °F) and 150 K (−123 °C/−189.4 °F), experiments find only crystal ice.
Droplets of supercooled water often exist in stratus and cumulus clouds. An aircraft flying through such a cloud sees an abrupt crystallization of these droplets, which can result in the formation of ice on the aircraft's wings or blockage of its instruments and probes, unless the aircraft is equipped with an appropriate de-icing system. Freezing rain is also caused by supercooled droplets.
The process opposite to supercooling, the melting of a solid above the freezing point, is much more difficult, and a solid will almost always melt at the same temperature for a given pressure. For this reason, it is the melting point which is usually identified, using melting point apparatus; even when the subject of a paper is "freezing-point determination", the actual methodology is "the principle of observing the disappearance rather than the formation of ice".[7] It is possible, at a given pressure, to superheat a liquid above its boiling point without it becoming gaseous.
Supercooling is often confused with freezing-point depression. Supercooling is the cooling of a liquid below its freezing point without it becoming solid. Freezing point depression is when a solution can be cooled below the freezing point of the corresponding pure liquid due to the presence of the solute; an example of this is the freezing point depression that occurs when salt is added to pure water.
Constitutional supercooling
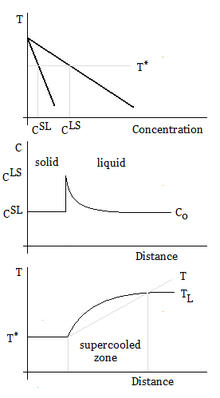
Constitutional supercooling, which occurs during solidification, is due to compositional solid changes, and results in cooling a liquid below the freezing point ahead of the solid–liquid interface. When solidifying a liquid, the interface is often unstable, and the velocity of the solid–liquid interface must be small in order to avoid constitutional supercooling.
Supercooled zones are observed when the liquidus temperature gradient at the interface is larger than the temperature gradient.
or
The slope of the liquidus phase boundary on the phase diagram is
The concentration gradient is related to points, and , on the phase diagram:
For steady-state growth and the partition function can be assumed to be constant. Therefore, the minimum thermal gradient necessary to create a stable solid front is as expressed below.
For more information, see the equation (3) of[8]
In animals
In order to survive extreme low temperatures in certain environments, some animals use the phenomenon of supercooling that allow them to remain unfrozen and avoid cell damage and death. There are many techniques that aid in maintaining a liquid state, such as the production of antifreeze proteins, or AFPs, which bind to ice crystals to prevent water molecules from binding and spreading the growth of ice.[3] The winter flounder is one such fish that utilizes these proteins to survive in its frigid environment. Noncolligative proteins are secreted by the liver into the bloodstream.[9] Other animals use colligative antifreezes, which increases the concentration of solutes in their bodily fluids, thus lowering their freezing point. Fish that rely on supercooling for survival must also live well below the water surface, because if they came into contact with ice nuclei they would freeze immediately. Animals that undergo supercooling to survive must also remove ice-nucleating agents from their bodies because they act as a starting point for freezing. Supercooling is also a common feature in some insect, reptile, and other ectotherm species. The potato cyst nematode larva (Globodera rostochiensis) could survive inside their cysts in a supercooled state to temperatures as low as −38 °C (−36 °F), even with the cyst encased in ice.
Supercooling is a last resort for animals. The best option is to move to a warmer environment if possible. As an animal gets farther and farther below its original freezing point the chance of spontaneous freezing increases dramatically for its internal fluids, as this is a thermodynamically unstable state. The fluids eventually reach the supercooling point, which is the temperature at which the supercooled solution freezes spontaneously due to being so far below its normal freezing point.[10] Animals unintentionally undergo supercooling and are only able to decrease the odds of freezing once supercooled. Even though supercooling is essential for survival, there are many risks associated with it.
In plants
As demonstrated by animals, plants can also survive extreme cold conditions brought forth during the winter months. Many plant species located in northern climates can acclimate under these cold conditions by supercooling, thus these plants survive temperatures as low as −40 °C. Although this supercooling phenomenon is poorly understood, it has been recognized through infrared thermography. Ice nucleation occurs in certain plant organs and tissues, debatably beginning in the xylem tissue and spreading throughout the rest of the plant.[11][12] Infrared thermography allows for droplets of water to be visualized as they crystalize in extracellular spaces.[13]
Supercooling inhibits the formation of ice within the tissue by ice nucleation and allows the cells to maintain water in a liquid state and further allows the water within the cell to stay separate from extracellular ice.[13] Cellular barriers such as lignin, suberin and the cuticle inhibit ice nucleators and force water into the supercooled tissue.[14] The xylem and primary tissue of plants are very susceptible to cold temperatures because of the large proportion of water in the cell. Many boreal hardwood species in northern climates have the ability to prevent ice spreading into the shoots allowing the plant to tolerate the cold.[15] Supercooling has been identified in the evergreen shrubs Rhododendron ferrugineum and Vaccinium vitis-idaea as well as Abies, Picea and Larix species.[15] Freezing outside of the cell and within the cell wall does not affect the survival of the plant.[16] However, the extracellular ice may lead to plant dehydration.[12]
Applications
One commercial application of supercooling is in refrigeration. Freezers can cool drinks to a supercooled level[17] so that when they are opened, they form a slush. Another example is a product that can supercool the beverage in a conventional freezer.[18] The Coca-Cola Company briefly marketed special vending machines containing Sprite in the UK, and Coke in Singapore, which stored the bottles in a supercooled state so that their content would turn to slush upon opening.[19]
Supercooling was successfully applied to organ preservation at Massachusetts General Hospital/Harvard Medical School. Livers that were later transplanted into recipient animals were preserved by supercooling for up to 96 hours (4 days), quadrupling the limits of what could be achieved by conventional liver preservation methods. The livers were supercooled to a temperature of –6 °C in a specialized solution that protected against freezing and injury from the cold temperature.[20]
Another potential application is drug delivery. In 2015, researchers crystallized membranes at a specific time. Liquid-encapsulated drugs could be delivered to the site and, with a slight environmental change, the liquid rapidly changes into a crystalline form that releases the drug.[21]
In 2016, a team at Iowa State University proposed a method for "soldering without heat" by using encapsulated droplets of supercooled liquid metal to repair heat sensitive electronic devices.[22][23] In 2019, the same team demonstrated the use of undercooled metal to print solid metallic interconnects on surfaces ranging from polar (paper and Jello) to superhydrophobic (rose petals), with all the surfaces being lower modulus than the metal.[24][25]
Eftekhari et al. proposed an empirical theory explaining that supercooling of ionic liquid crystals can build ordered channels for diffusion for energy storage applications. In this case, the electrolyte has a rigid structure comparable with that of a solid electrolyte, but the diffusion coefficient can be as large as in liquid electrolytes. Supercooling increases the medium viscosity but keeps the directional channels open for diffusion.[26]
See also
References
- O. Gomes, Gabriel; Stanley, H. Eugene; Souza, Mariano de (2019-08-19). "Enhanced Grüneisen Parameter in Supercooled Water". Scientific Reports. 9 (1): 12006. arXiv:1808.00536. Bibcode:2019NatSR...912006O. doi:10.1038/s41598-019-48353-4. ISSN 2045-2322. PMC 6700159. PMID 31427698.
- Rathz, Tom. "Undercooling". NASA. Archived from the original on 2009-12-02. Retrieved 2010-01-12. Cite journal requires
|journal=(help)CS1 maint: ref=harv (link) - J.G. Duman (2001). "Antifreeze and ice nucleator proteins in terrestrial arthropods". Annual Review of Physiology. 63: 327–357. doi:10.1146/annurev.physiol.63.1.327. PMID 11181959.
- Moore, Emily; Valeria Molinero (24 November 2011). "structural transformation in supercooled water controls the crystallization rate of ice". Nature. 479 (7374): 506–508. arXiv:1107.1622. Bibcode:2011Natur.479..506M. doi:10.1038/nature10586. PMID 22113691.
- Debenedetti, P. G.; Stanley, H. E. (2003). "Supercooled and Glassy Water" (PDF). Physics Today. 56 (6): 40–46 [p. 42]. Bibcode:2003PhT....56f..40D. doi:10.1063/1.1595053.CS1 maint: ref=harv (link)
- Angell, C. Austen (2008). "Insights into Phases of Liquid Water from Study of Its Unusual Glass-Forming Properties". Science. 319 (5863): 582–587. doi:10.1126/science.1131939.
- Ramsay, J. A. (1949). "A new method of freezing-point determination for small quantities" (PDF). J. Exp. Biol. 26 (1): 57–64.
- page from 99~100 Archived July 29, 2013, at the Wayback Machine
- Garth L Fletcher; Choy L Hew & Peter L Davies (2001). "Antifreeze Proteins of Teleost Fishes". Annual Review of Physiology. 63: 359–390. doi:10.1146/annurev.physiol.63.1.359. PMID 11181960.
- C.H. Lowe; P.J. Lardner & E.A. Halpern (1971). "Supercooling in reptiles and other vertebrates". Comparative Biochemistry and Physiology. 39A (1): 125–135. doi:10.1016/0300-9629(71)90352-5. PMID 4399229.
- Wisniewski, M (1997). "Observations of ice nucleation and propagation in plants using infrared thermography". Plant Physiology. 113 (2): 327–334. doi:10.1104/pp.113.2.327. PMC 158146. PMID 12223611.
- Pearce, R (2001). "Plant freezing and damage" (PDF). Annals of Botany. 87 (4): 417–424. doi:10.1006/anbo.2000.1352. Retrieved 11 December 2016.
- Wisniewski, M (2004). "Ice nucleation, propagation, and deep supercooling in woody plants". Journal of Crop Improvement. 10 (1–2): 5–16. doi:10.1300/j411v10n01_02.
- Kuprian, E (2016). "Persistent supercooling of reproductive shoots is enabled by structural ice barriers being active despite intact xylem connection". PLOS One. 11 (9): e0163160. Bibcode:2016PLoSO..1163160K. doi:10.1371/journal.pone.0163160. PMC 5025027. PMID 27632365.
- Neuner, Gilbert (2014). "Frost resistance in alpine woody plants". Front Plant Sci. 5: 654. doi:10.3389/fpls.2014.00654. PMC 4249714. PMID 25520725.
- Burke, M (1976). "Freezing and injury in plants". Annual Review of Plant Physiology. 27: 507–528. doi:10.1146/annurev.pp.27.060176.002451.
- Chill Chamber Archived March 1, 2009, at the Wayback Machine
- Slush-It! Archived 2010-01-23 at the Wayback Machine
- Charlie Sorrel (2007-09-21). "Coca Cola Plans High Tech, Super Cool Sprite". Wired. Condé Nast. Retrieved 2013-12-05.
- Berendsen, TA; Bruinsma, BG; Puts, CF; Saeidi, N; Usta, OB; Uygun, BE; Izamis, Maria-Louisa; Toner, Mehmet; Yarmush, Martin L; Uygun, Korkut (2014). "Supercooling enables long-term transplantation survival following 4 days of liver preservation". Nature Medicine. 20 (7): 790–793. doi:10.1038/nm.3588. PMC 4141719. PMID 24973919.
- Hunka, George (2015-05-06). "A "super cool" way to deliver drugs". R&D.
- Mitch Jacoby (2016-03-14). "Soldering without heat". Chemical and Engineering News. Retrieved 2016-03-14.
- Simge Çınar, Ian D. Tevis, Jiahao Chen & Martin Thuo (2016-02-23). "Mechanical Fracturing of Core-Shell Undercooled Metal Particles for Heat-Free Soldering". Nature. Retrieved 2016-03-14.CS1 maint: multiple names: authors list (link)
- Mitch Jacoby (2019-07-23). "Heat-free method yields printed metallic circuit connections". Chemical and Engineering News. Retrieved 2019-07-24.
- Andrew Martin; Boyce S. Chang; Zachary Martin; Dipark Paramanik; Christophe Frankiewicz; Souvik Kundu; Ian Tevis; Martin Thuo (2019-07-15). "Heat-Free Fabrication of Metallic Interconnects for Flexible/Wearable Devices". Advanced Functional Materials. 29 (40): 1903687. doi:10.1002/adfm.201903687.
- Eftekhari, A; Liu, Y; Chen, P (2016). "Different roles of ionic liquids in lithium batteries". Journal of Power Sources. 334: 221–239. Bibcode:2016JPS...334..221E. doi:10.1016/j.jpowsour.2016.10.025.
Further reading
- Giovambattista, N.; Angell, C. A.; Sciortino, F.; Stanley, H. E. (July 2004). "Glass-Transition Temperature of Water: A Simulation Study" (PDF). Physical Review Letters. 93 (4): 047801. arXiv:cond-mat/0403133. Bibcode:2004PhRvL..93d7801G. doi:10.1103/PhysRevLett.93.047801. PMID 15323794.CS1 maint: ref=harv (link)
- Rogerson, M. A.; Cardoso, S. S. S. (April 2004). "Solidification in heat packs: III. Metallic trigger". AIChE Journal. 49 (2): 522–529. doi:10.1002/aic.690490222.CS1 maint: ref=harv (link)