Tetradentate ligand
Tetradentate ligands are ligands that bind with four donor atoms to a central atom to form a coordination complex. This number of donor atoms that bind is called denticity and is a way to classify ligands. Tetradentate ligands are common in nature in the form of chlorophyll which has a core ligand called chlorin, and heme with a core ligand called porphyrin. They add much of the colour seen in plants and humans. Phthalocyanine is an artificial macrocyclic tetradentate ligand that is used to make blue and green pigments.
Shape
Tetradentate ligands can be classified by the topology of the connections between the donor atoms. Common forms are linear (also called sequential), ring or tripodal. A tetrapodal ligand that is also tetradentate has four legs with donor atoms, and a bridgehead that is not a donor. On binding with a central atom there are several arrangements possible called geometric isomers.
Linear ligands
A linear tetradentate ligand has the four donor atoms in a line, each subsequent donor is connected by one of three bridges.
A linear tetradetate ligand bound to a metal in tetrahedral coordination can only connect in one way. If the ligand is unsymmetrical then there are two chiral arrangements.
A linear tetradetate ligand bound to a metal in square planar coordination in one way. Anticlockwise or clockwise arrangements are equivalent.
Linear ligands in octahedral coordination
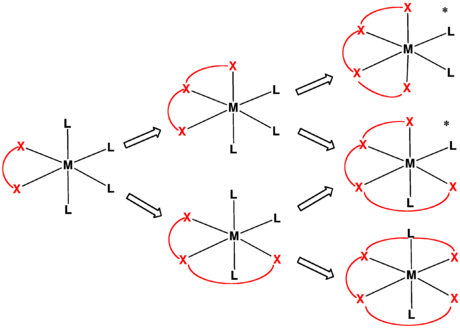
A linear tetradentate ligand has its donor atoms arranged along or in a chain, so that each adjacent donor atom has to be adjacent on the central atom. This arrangement leads to three stereochemical outcomes. The four donor groups can be co-equatorial. This geometry is called trans because the remaining unoccupied positions on the octahedron are mutually trans (opposite).[1][2][3] When the two internal donor atoms, e.g. the secondary amines in trien or EDDA, are pyramidal, two diastereomers for the trans arrangement are determined by the relative stereochemistry of these centers. Typically these donors are mutually trans, resulting in a chiral complex of C2-symmetric complexes. This arrangement is illustrated by complexes of the Trost ligand.
The ligand can have one bend so that one is at the pole and three on the equator of the central atom. This is called beta cis-β (beta). The remaining octahedral positions are cis (adjacent) to each other. The triangles of coordinating atoms and the central atom have two coplanar, and one perpendicular. This arrangement is chiral, so there are two possible mirror images. The arrangement where the chain goes down and clockwise is termed lambda Λ, and where is goes down and anticlockwise is called delta Δ.[2] If the chain is not symmetrical, then different isomers can be produced by which end of the ligand has the bend. If three donor atoms are the same at one end of the chain, the mer- and fac- prefixes used for tridentate ligands can be used giving β-mer- if the three are arranged on a meridian or β-fac if the three are arranged on a face of the octahedron.[1]
The chain can have two bends, so there is one at a pole, two on the equator and one at the opposite pole. None of the triangles of coordinating atoms and the central atom are coplanar. This is termed cis-α (alpha). This arrangement is chiral, so there are two possible mirror images. The arrangement where the chain goes down and clockwise and down is termed lambda Λ, and where is goes down and anticlockwise and down is called delta Δ.[4][2]
Tripodal ligands
Tripodal tetradentate ligands have a donor atom connected via three chains to other donor atoms. The top of the tripod is called the apex, and a donor atom in that position is apical, or also known as the bridging atom. The other three donor atoms are on the "feet" of the tripod. Examples can have three identical chains attached to an atom in tertiary arrangement. This atom could be nitrogen, phosphorus, or arsenic. Molecules containing phosphorus, or arsenic donor atoms remain stiff at the P or As and can hold their shape, unlike nitrogen compounds which rapidly racemize. If all the feet of the tripod are symmetrical and identical to each other, there will be only one way to attach in an octahedral coordination. However, there are two non-equivalent positions left on the central atom, so if two different monodentate, or an unsymmetrical bidentate ligand attaches there will be two possible isomers. If the feet differ then there are more isomers. When two feet are the same, and one is different there are three arrangements, two of which are enantiomers of each other. When there are three different legs, there are six possible isomers, but two are enantiomers of another pair, and two are symmetric.[5]
Atoms with five coordinate positions are usually trigonal bipyramidal or square pyramid in shape. A symmetric tripodal tetradentate ligand can form two isomers on a square pyramid, depending on whether the bridging donor is on the apex or the base of the pyramid. The extra vacant position on the square pyramid is on the base. Square pyramidal coordination tends to occur where a six-member ring is formed with the bridgehead, bridge, feet donor atom and central atom. The longer leg (with three bridging atoms) connects to the apex of the pyramid, and symmetry is lost.[6]
For the trigonal bipyramid, the tripod shaped ligand has its most symmetrical position with the bridging donor at one of the apexes, and the feet of the tripod are arranged around the base, leaving a vacant position at the opposite apex. This has C3v symmetry. Trigonal bipyramidal coordination tends to occur where five member rings are formed with the bridgehead, bridge, feet donor atoms and central atom.[6]
In four coordination a tripodal ligand would fill all the positions available, the geometry is trigonal pyramid. The shape is distorted from the tetrahedron due to the non-symmetry of the tripod.[6]
Classification
In addition to shape, tetradentate ligands can be classified by the ligating atoms on the ligand. For linear ligands the order can be given. The ligand may have a negative charge when it is in a complex with the central atom. This may develop through the loss of hydrogen ions when the substance is dissolved.
Other characteristics are the size of the rings formed by the central metal with two donor atoms and the intervening chain if the ligand. Usually these rings have five or six members, but sometimes seven atoms.[7] For ring shaped ligands, the total number of atoms in the ring is important, as it is a determiner of the hole size for the central atom.[7] Each additional atom in the ring enlarges the hole radius from 0.1 to 0.15 Å.[7]
Ligands are also characterised by charge. Tetradentate ligands can be neutral, so that the charge of the whole complex is the same as the central atom. A tetradentate monoanionic (TMDA) ligand has one donor atom with a negative charge.[8] A tetradentate dianionic ligand has a double negative charge, and tetradentate trianionic ligands have a triple negative charge. The maximal charge is on tetradentate tetraanionic ligands, which can stabilise metals in high oxidation states, however such ligands also have to resist oxidation to survive a strongly oxidising atom.[9]
List
| name | abbreviation | formula | shape | type | charge | MW | central atoms | pic |
|---|---|---|---|---|---|---|---|---|
| Chlorin | ring | NNNN | –2 | 312.3678 | Mg | 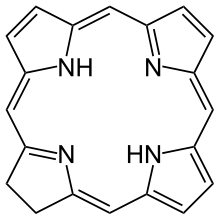 | ||
| Corrin | ring | NNNN | –1 | 306.40 | Co | 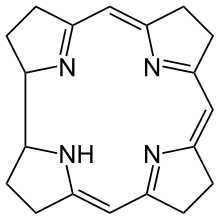 | ||
| 1,4,7,10-tetraoxacyclododecane | 12-crown-4 | (C2H4O)4 | ring | OOOO | 0 | 176.21 | Li | 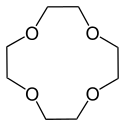 |
| 1,4,8,11-tetraazacyclotetradecane | cyclam | (NHCH2CH2NHCH2CH2CH2)2 | ring | NNNN | 200.33 | transition metals | 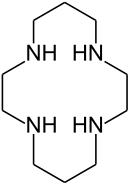 | |
| 1,4,7,10-tetraazacyclododecane | cyclen | ring | N4 | 172.271 | Zn | 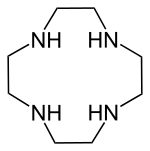 | ||
| Dibenzotetramethyltetraaza[14]annulene[10] | tmtaa | ring | NNNN | 2- | UO2 | |||
| N,N-ethylenediaminediacetic acid | NH2C2H4N(CH2COOH)2 | tripodal | NNO2 | 2– | ||||
| N,N'-ethylenediaminediacetic acid | (-CH2NHCH2COOH)2 | linear | ONNO | 2– | ||||
| N-hydroxyimino-2,2'-dipropionic acid | H3HIDPA | HON(CH(CH3)CO2H)2 | linear | ONOO | 3– | V4+ | 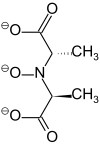 | |
| diethylenetriamineacetic acid | DTMA[1] | NH2C2H4NHC2H4NHCH2COOH | linear | NNNO | 1– | Co | ||
| iso-diethylenetriamineacetic acid | i-DTMA[1] | (NH2C2H4)2NCH2COOH | tripodal | NN2NO | 1– | Co | ||
| Jäger's N2O2 ligand | linear acacen | ONNO N2O2 | Ni | |||||
| Naphthalocyanine | C48H26N8 | ring | NNNN | 714.79 | 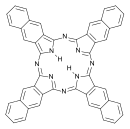 | |||
| Nitrilotriacetic acid | NTA | N(CH2CO2H)3 | tripodal | NO3 | 3– | 191.14 | Ca2+, Cr, Cu2+, and Fe3+, Ni | 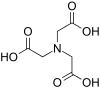 |
| Phthalocyanine | H2Pc | C32H18N8 | ring | NNNN | 2– | Cu, Co | 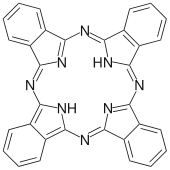 | |
| Porphyrin[11] | ring | NNNN | Mg, V, Fe, Ni | 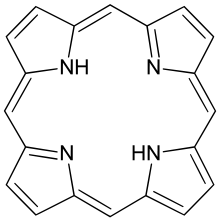 | ||||
| Rhodotorulic acid | C14H24N4O6 | I shape | OOOO | 344.36 | Fe3+ | 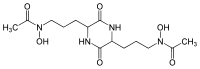 | ||
| Salen ligand | linear | ONNO N2O2 | 268.31 | |||||
| salpn ligand | salpn | linear | ONNO | 2− | 282.34 | Cr, Cu, Fe, Ni | 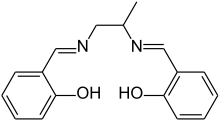 | |
| tetars (meso and racemic isomers)[12] | [(CH3)2As(CH2)3As(C6H5)CH2]2 | linear | AsAsAsAs | 0 | Co2+ | |||
| 1,1,4,7,10,10-hexaphenyl-1,4,7,10-tetraphosphadecane tetraphos |
tet-1 | linear | PPPP | 0 | 670.68 | Fe+ Ru+ Os+ Re3+[2] Pd2+ Pt2+ | ||
| 1,4,7,10-tetrathiadodecane[13] | [12]-ane-S4 | ring | SSSS | 0 | Cu2+ | |||
| 1,4,7,10-tetrathiatridecane[13] | [13]-ane-S4 | ring | SSSS | 0 | Cu2+ | |||
| 1,4,8,11-tetrathiatetradecane[13] | [14]-ane-S4 | ring | SSSS | 0 | Cu2+ | |||
| 1,4,8,12-tetrathiapentadecane[13] | [15]-ane-S4 | ring | SSSS | 0 | Cu2+ | |||
| 1,5,9,13-tetrathiahexadecane[13] | [14]-ane-S4 | ring | SSSS | 0 | Cu2+ | |||
| 2,5,8-trithia[9](2,5)thiophenophane [13] | ring | SSSS | 0 | Cu2+ | ||||
| Triethylene glycol dimethyl ether | TG3 | CH3(OCH2CH2)3OCH3 | linear | OOOO | 0 | 178.23 | neutral Na, K[14] | |
| Triethylenetetramine | TETA trien |
[CH2NHCH2CH2NH2]2 | linear | NNNN | 146.24 | Cu2+ | ||
| tris-(dimethylarsinopropyl)-arsine[15] | As[CH2CH2CH2As(CH3)2]3 | tripod | AsAs3 | 0 | Fe2+ Ni2+ Co3+ oct Ni3+ tbp | |||
| tris-(o-dimethylarsinophenyl)-arsine[15] | As[o-C6H4As(CH3)2]3 | tripod | AsAs3 | 0 | Pt2+ Pd2+ Ni2+ tbp Ru2+ oct | |||
| tris-(o-diphenylarsinophenyl)-arsine[15] | As[o-C6H4As(C6H5)2]3 | tripod | AsAs3 | 0 | Pt2+ Pd2+ Ru0 Rh+ Ni2+ tbp Re2+ Ru2+ Os2+ Rh3+ Pd4+ Pt4+ oct | |||
| CH3[As(CH3)o-C6H4]3AsCH(3)2[15] | linear | As4 | 0 | Pd2+ square pyramydal | ||||
| [As(C6H5)2o-C6H4As(C6H5)CH2]2[15] | linear | As4 | 0 | Ni2+ 4 coordinate Ni2+ Co2+ five coorinate | ||||
| tris-(o-diphenylphosphinophenyl)-phosphine[15] | tripod tetraphosphine of Venanzi | P[o-C6H4P(C6H5)2]3 | tripod | PP3 | 0 | Pd2+ Pt2+ Ru0 Ru2+ Os2+ Cr0 Cr+ Cr3+ Mn+ Co3+ oct Ni2+ Fe2+ Co+ Co2+ tbp | ||
| Tris(2-pyridylmethyl)amine | TPA | tripodal | N3N | 290.37 | Cu | amine_(structural_diagram).png) | ||
Biomolecules
Heme is a heterocyclic macrocycle ring shaped tetradentate ligand. It is an important molecule in red blood cells.
Chlorophyll comes in several forms and is important in plant photosynthesis. Bacteria may use variants called bacteriochlorophylls.
References
- Schneider, Peter W.; Collman, James P. (October 1968). "Complexes of cobalt(III) with the two isomeric diethylenetriamineacetic acids". Inorganic Chemistry. 7 (10): 2010–2015. doi:10.1021/ic50068a010.
- Bautista, Maria Teresa; Earl, Kelly Anne; Maltby, Patricia Anne; Morris, Robert Harold; Schweitzer, Caroline Theresia (March 1994). "New dihydrogen complexes: the synthesis and spectroscopic properties of iron(II), ruthenium(II), and osmium(II) complexes containing the meso-tetraphos-1 ligand". Canadian Journal of Chemistry. 72 (3): 547–560. doi:10.1139/v94-078.
- Sloan, Thomas E.; Busch, Daryle H. (27 May 1980). "Stereochemical Description and Notation for Coordination Systems". Stereochemistry of Optically Active Transition Metal Compounds. p. 401. doi:10.1021/bk-1980-0119.ch021.
- "Coordination Chemistry I Structures and Isomers" (PDF). p. 51.
- KOINE, Norio; YAMAGUCHI, Hiroyuki; TANIGAKI, Teiichi; HIDAKA, Jinsai; SHIMURA, Yoichi (1974). "PREPARATION AND STRUCTURAL ASSIGNMENT OF ISOMERS OF POTASSIUM TRANS(N)- (C-METHYL SUBSTITUTED AMMONIATRIACETATO) (β-ALANINATO) COBALTATE(III)". Chemistry Letters. 3 (9): 993–996. doi:10.1246/cl.1974.989. open access
- Ambundo, Edna A.; Yu, Qiuyue; Ochrymowycz, L. A.; Rorabacher, D. B. (August 2003). "Electron-Transfer Kinetics of Copper(II/I) Tripodal Ligand Complexes". Inorganic Chemistry. 42 (17): 5267–5273. doi:10.1021/ic030176c.
- Lindoy, Leonard F. (1989). The chemistry of macrocyclic ligand complexes (1. publ., transferred to digital print. ed.). Cambridge [England]: Cambridge University Press. p. 4. ISBN 052125261X.
- Chomitz, WayneA.; Arnold, John (16 February 2009). "Use of Tetradentate Monoanionic Ligands for Stabilizing Reactive Metal Complexes". Chemistry - A European Journal. 15 (9): 2020–2030. doi:10.1002/chem.200801069.
- Collins, Terrence J.; Kostka, Kimberly L.; Munck, Eckard; Uffelman, Erich S. (July 1990). "Stabilization of mononuclear five-coordinate iron(IV)". Journal of the American Chemical Society. 112 (14): 5637–5639. doi:10.1021/ja00170a037.
- Pedrick, Elizabeth A.; Assefa, Mikiyas K.; Wakefield, Megan E.; Wu, Guang; Hayton, Trevor W. (15 May 2017). "Uranyl Coordination by the 14-Membered Macrocycle Dibenzotetramethyltetraaza[14]annulene". Inorganic Chemistry. 56 (11): 6638. doi:10.1021/acs.inorgchem.7b00700. PMID 28504885.
- Numerous derivatives of porpyrin are known. See the list in Buchler, J. W.; Dreher, C.; Kunzel, F. M. (1995). Buchler, J. W. (ed.). Metal complexes with tetrapyrrole ligands III. Berlin: Springer. p. 5. ISBN 978-3-540-59281-5.
- Bosnich, B.; Jackson, W. G.; Wild, S. B. (December 1973). "Metal complexes of dissymmetric arsines. Stereochemistry, topological stability, and spectra of cobalt(III) complexes containing a linear quadridenate tetra-tert-arsine ligand". Journal of the American Chemical Society. 95 (25): 8269–8280. doi:10.1021/ja00806a012.
- Zanello, P. (1990). "ELECTROCHEMISTRY OF MONONUCLEAR COPPER COMPLEXES. STRUCTURAL REORGANIZATIONS ACCOMPANYING REDOX CHANGES". In Bernal, Ivan (ed.). Stereochemical control, bonding, and steric rearrangements. Amsterdam: Elsevier. p. 208. ISBN 0444888411.
- Down, J. L.; Lewis, J.; Moore, B.; Wilkinson, G. (1959). "The solubility of alkali metals in ethers". Journal of the Chemical Society: 3767. doi:10.1039/JR9590003767.
- McAuliffe, C. A. (1973). Transition Metal Complexes of Phosphorus, Arsenic and Antimony Ligands. Macmillan Ecucation. pp. 282–285. ISBN 9780333136287.