Etretinate
Etretinate (trade name Tegison) is a medication developed by Hoffmann–La Roche that was approved by the FDA in 1986 to treat severe psoriasis. It is a second-generation retinoid.[1] It was subsequently removed from the Canadian market in 1996 and the United States market in 1998 due to the high risk of birth defects. It remains on the market in Japan as Tigason.
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Tigason, formerly Tegison |
| AHFS/Drugs.com | Drugs.com archive |
| MedlinePlus | a601010 |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | >99% |
| Metabolites | Free acid, Z-form, chain shortening |
| Elimination half-life | 120 days |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.053.727 |
| Chemical and physical data | |
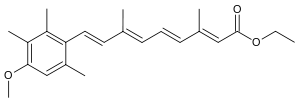
| Formula | C23H30O3 |
| Molar mass | 354.490 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Pharmacology
Etretinate is a highly lipophilic, aromatic retinoid. It is stored and released from adipose tissue, so its effects can continue long after dosage stops. It is detectable in the plasma for up to three years following therapy. Etretinate has a low therapeutic index and a long elimination half-life (t1/2) of 120 days,[1] which make dosing difficult.
Etretinate has been replaced by acitretin, the free acid (without the ethyl ester). While acitretin is less lipophilic and has a half-life of only 50 hours, it is partly metabolized to etretinate in the body,[1] so that it is still a long-acting teratogen and pregnancy is prohibited for two years after therapy.[2]
Precautions
- Etretinate is a teratogen, and may cause birth defects long after use. Therefore, birth control is advised during therapy, and for at least three years after therapy has stopped.[3]
- Etretinate should be avoided in children, as it may interfere with bone growth.[3]
- If a patient has ever taken etretinate, he or she is not eligible to donate blood in the United States, the United Kingdom, or Québec, due to the risk of birth defects.[4][5] In Japan, people may not donate blood for two years after ceasing to use the medication.[6]
Side effects
Side effects are those typical of hypervitaminosis A, most commonly[3]
- bone or joint pain, stiffness; in long-term treatment diffuse idiopathic skeletal hyperostosis
- muscular or abdominal cramps
- dry, burning, itching eyelids
- unusual bruising
History
The drug was approved by the FDA in 1986 to treat severe psoriasis. It was subsequently removed from the Canadian market in 1996 and the United States market in 1998 due to the high risk of birth defects.[3][7][8]
In Japan, the drug remains on market branded Tigason.[6]
See also
References
- Mutschler E, Schäfer-Korting M (2001). Arzneimittelwirkungen (in German) (8 ed.). Stuttgart: Wissenschaftliche Verlagsgesellschaft. p. 728f. ISBN 3-8047-1763-2.
- Jasek W, ed. (2007). Austria-Codex (in German) (62nd ed.). Vienna: Österreichischer Apothekerverlag. p. 5669. ISBN 978-3-85200-181-4.
- Drugs.com archive for etretinate
- "Donor Selection Guidelines: Etretinate". UK Blood Transfusion and Tissue Transplantation Services.
- "Medications taken on a regular basis that exclude you from donating blood". Héma-Québec.
- "Tigason Drug information sheet". RAD-AR Council Japan. Archived from the original on 27 January 2013.
- Qureshi ZP, Seoane-Vazquez E, Rodriguez-Monguio R, Stevenson KB, Szeinbach SL (July 2011). "Market withdrawal of new molecular entities approved in the United States from 1980 to 2009". Pharmacoepidemiology and Drug Safety. 20 (7): 772–7. doi:10.1002/pds.2155. PMID 21574210.
- Fung M, Thornton A, Mybeck K, Wu JH, Hornbuckle K, Muniz E (1 January 2001). "Evaluation of the Characteristics of Safety Withdrawal of Prescription Drugs from Worldwide Pharmaceutical Markets-1960 to 1999". Therapeutic Innovation & Regulatory Science. 35 (1): 293–317. doi:10.1177/009286150103500134.