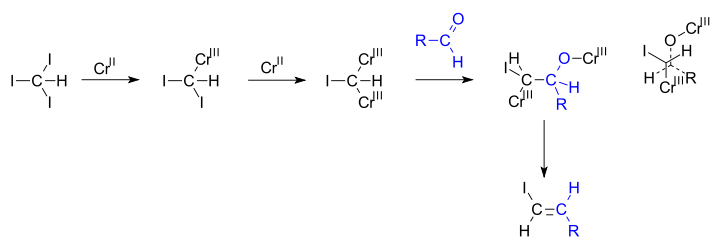
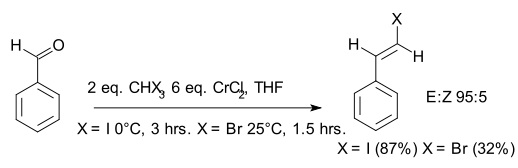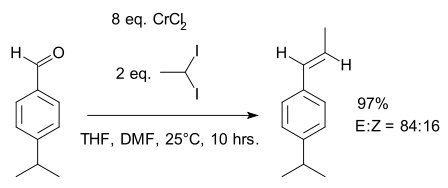Takai olefination
Takai olefination in organic chemistry describes the organic reaction of an aldehyde with a diorganochromium compound to form an alkene. In the original 1986 publication[1] the aldehyde is benzaldehyde and the organochromium species is generated from iodoform or bromoform and an excess of chromium(II) chloride. The reaction product is a vinyl halide. The main selling point is the E-configuration of the double bond. According to the principal investigator Kazuhiko Takai, existing alternatives such as the Wittig reaction only yield mixtures.
| Takai olefination | |
|---|---|
| Named after | Kazuhiko Takai |
| Reaction type | Carbon-carbon bond forming reaction |
In the reaction mechanism proposed by Takai, chromium(II) is oxidized to chromium(III) eliminating two equivalents of a halide. The geminal carbodianion complex thus formed (determined as [Cr2Cl4(CHI)(THF)4])[2][3] reacts with the aldehyde in a 1,2-addition along one of the carbon to chromium bonds and in the next step both chromium bearing groups engage in an elimination reaction. In newman projection it can be seen how the steric bulks of chromium groups and the steric bulks of the alkyl and halogen groups drive this reaction towards anti elimination.[4]
History of the reaction
Kazuhiko Takai is a professor of applied chemistry at Okayama University and is the recipient of the 2013 Chemical Society of Japan Award.[1][5] He primarily studies the use catalytic metals in synthesis reactions. He studied at Kyoto University and the University of California, Berkeley. The Takai olefination reaction was first described in a 1986 paper in the Journal of the American Chemical Society. Written by Kazuhiko Takai, Kenji Nitta, and Kiitiro Utimoto, the paper discusses the limitations of existing reagents, such as the Wittig reagent, in creating solely E isomers.[6][1] Nitta, a graduate student working in the Utimoto group, carried out the reaction.
Similar olefination reactions have been performed, using a variety of reagents such as zinc and lead chloride;[7] however, these olefination reactions often lead to the formation of McMurry diols, not the methylenation or alkylidenation of aldehydes.[8] To circumvent this issue, Takai researched the potential of chromium (II) salts.
The reaction primarily employs the use of aldehydes, but ketones may be used. However, ketones do not react as well as aldehydes; thus, for a compound with both aldehyde and ketone groups, the reaction can target just the aldehyde group and leave the ketone group intact.[1]
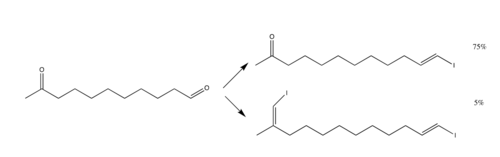
The drawbacks to the reaction include the fact that stoichiometrically, 4 equivalents of chromium chloride must be used, since there is a reduction of two halogen atoms.[3] Ways to limit the amount of chromium chloride exist, namely by utilization of zinc equivalent,[9] but this method remains unpopular.
Takai–Utimoto olefination
In a second publication the scope of the reaction was extended to diorganochromium intermediates bearing alkyl groups instead of halogens:[10]
References
- Takai, K.; Nitta, K.; Utimoto, K. (1986). "Simple and selective method for RCHO → (E)-RCH==CHX conversion by means of a CHX3–CrCL2 system". Journal of the American Chemical Society. 108 (23): 7408–7410. doi:10.1021/ja00283a046.
- Werner, Daniel; Anwander, Reiner (28 September 2018). "Unveiling the Takai Olefination Reagent via Tris(tert-butoxy)siloxy Variants". Journal of the American Chemical Society. 140 (43): 14334–14341. doi:10.1021/jacs.8b08739. ISSN 0002-7863. PMID 30213182.
- Murai, Masahito; Taniguchi, Ryuji; Hosokawa, Naoki; Nishida, Yusuke; Mimachi, Hiroko; Oshiki, Toshiyuki; Takai, Kazuhiko (2017). "Structural Characterization and Unique Catalytic Performance of Silyl-Group-Substituted Geminal Dichromiomethane Complexes Stabilized with a Diamine Ligand". Journal of the American Chemical Society. 139 (37): 13184–13192. doi:10.1021/jacs.7b07487. PMID 28814078.
- Kürti, László; Czakó, Barbara (2005). Strategic Applications of Named Reactions in Organic Synthesis. Burlington; San Diego; London: Elsevier Academic Press. ISBN 978-0-12-369483-6.
- "Development of Novel Synthetic Organic Reactions by the Use of Group 4-7 Metals". The Chemical Society of Japan. Retrieved 2020-04-30.
- achem.okayama-u.ac.jp http://achem.okayama-u.ac.jp/omc/cv-takai-e.html. Retrieved 2020-04-13.
- Okazoe, Takashi; Takai, Kazuhiko; Oshima, Koichiro; Utimoto, Kiitiro (1987). "Alkylidenation of ester carbonyl groups by means of a reagent derived from RCHBr2, Zn, TiCl4, and TMEDA. Stereoselective preparation of (Z)-alkenyl ethers". The Journal of Organic Chemistry. 52 (19): 4410–4412. doi:10.1021/jo00228a055.
- Mukaiyama, Teruaki; Sato, Toshio; Hanna, Junichi (1973). "Reductive coupling of carbonyl compounds to pinacols and olefins by using TiCl4 and Zn". Chemistry Letters. 2 (10): 1041–1044. doi:10.1246/cl.1973.1041.
- Takai, Kazuhiko; Ichiguchi, Tetsuya; Hikasa, Shintaro (1999). "A Practical Transformation of Aldehydes into (E)-Iodoalkenes with Geminal Dichromium Reagents". Synlett. 1999 (8): 1268–1270. doi:10.1055/s-1999-2829.
- Okazoe, T.; Takai, Kazuhiko; Utimoto, K. (1987). "(E)-Selective olefination of aldehydes by means of gem-dichromium reagents derived by reduction of gem-diiodoalkanes with chromium(II) chloride". Journal of the American Chemical Society. 109 (3): 951–953. doi:10.1021/ja00237a081.