TSBP1
TSBP1 is a protein that in humans is encoded by the TSBP1 gene.[3][4] C6orf10 is an open reading frame on chromosome 6 containing a protein that is ubiquitously expressed at low levels in the adult genome and may play a role during fetal development. C6orf10 has been found to be linked to both neurodegenerative and autoimmune diseases in adults. Expression of this gene is highest in the testis but is also seen in other tissue types such as the brain, lens of the eye and the medulla.[5][6][7] TSBP1 was previously known as C6orf10.
| TSBP1 | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||||||||||||||||||
| Aliases | TSBP1, TSBP, chromosome 6 open reading frame 10, testis expressed basic protein 1, C6orf10 | ||||||||||||||||||||||||
| External IDs | OMIM: 618151 HomoloGene: 81753 GeneCards: TSBP1 | ||||||||||||||||||||||||
| |||||||||||||||||||||||||
| |||||||||||||||||||||||||
| Orthologs | |||||||||||||||||||||||||
| Species | Human | Mouse | |||||||||||||||||||||||
| Entrez |
| ||||||||||||||||||||||||
| Ensembl |
|
| |||||||||||||||||||||||
| UniProt |
| ||||||||||||||||||||||||
| RefSeq (mRNA) |
| ||||||||||||||||||||||||
| RefSeq (protein) |
| ||||||||||||||||||||||||
| Location (UCSC) | Chr 6: 32.29 – 32.37 Mb | n/a | |||||||||||||||||||||||
| PubMed search | [2] | n/a | |||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||
| |||||||||||||||||||||||||
Protein
Composition
C6orf10 isoform a is rich in lysine (K), Glutamine (Q) and Glutamic acid (E) and poor in Histidine (H) and Phenylalanine(F)[11]]. Isoform a is a basic protein with an isoelectric point of 9 and a molecular weight of 62,000 kDa.[12]
This isoform contains two transmembrane regions near the beginning of the amino acid sequence.[13] The first transmembrane region spans from residue 6 to residue 25 (19 total residues) and has an isoelectric point of 5. The second transmembrane region spans from residue 100 to residue 119 (19 total residues) and has an isoelectric point of 8. Isoform a contains a PTZ00121 domain[14] starting with residue 221 and going until the end of the protein. There are several repetitive sequences within this domain.
Secondary Structure
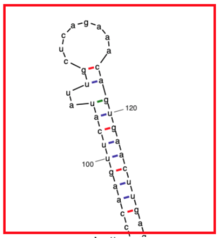
TSBP1 consists mostly of alpha helices and random coils.[15] There are only a few regions that contain beta sheets.[16][17]
Subcellular Localization
TSBP1 is predicted to be localized to the Nucleus and the Endoplasmic Reticulum.[13] There is a signal peptide cleavage site between amino acid 30 and 31[18] which includes the first transmembrane domain. This N-terminal region of C6orf10 is likely localized to the endoplasmic reticulum. The C-terminal region of the protein contain two nuclear localization signals from amino acid 489-505 and 513-529 indicating that the section of the protein after the signal peptide cleavage site is localized to the nucleus.
Expression
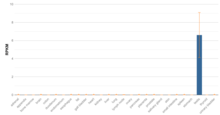
TSBP1 is ubiquitously expressed at low levels in the adult human genome. In adults, expression of this gene is highest in the testis.[19] C6orf10 is expressed at higher levels in fetal and embryonic tissues. This indicates C6orf10 may play a role in development.

Regulation of expression
Transcriptional
TSBP1 has a promoter that is 1206 bases long.[20] This promoter overlaps with the 3' UTR but ends before the first codon. This promoter is fairly well conserved across primates except for a 136 nucleotide region midway through and the end of the promoter region.[21] Primates have insertions at these two regions that humans are missing. This may suggest that these regions of the promoter are not essential to humans.
Transcription factors
TSBP1 transcription is regulated by the binding of many transcription factors to the promoter region. The CCAAT binding protein and TATA box are highly conserved regions that are important in the initiation of transcription. Several of the transcription factors including EH1, NACA, NKX5-2, SIX4, VCR, etc. are involved in developmental pathways.[20]
| Abbreviation | Transcription Factor Full Name | Matrix score | Strand |
| CSRNP-1 | Cytosine-Serine rich nuclear protein 1(AXUD1, AXIN1 up-regulated-1) | 1.0 | + |
| CCAAT Box | CCAAT/enhancer binding protein (C/EBP), gamma | 0.923 | + |
| EH1 | Engrailed Homeobox 1 | 0.862 | + |
| Cart-1 | Cartilage homeoprotein 1 | 0.997 | - |
| ZFP 263 | Zinc finger protein 263, ZKSCAN12 (Zinc finger protein with KRAB and SCAN domains 12) | 0.921 | + |
| SWI/SNF | SWI/SNF related, matrix associated, actin dependent regulator of chromatin, subfamily, a member 3 | 0.999 | - |
| TATA Box | Vertebrate TATA binding protein factor | 0.899 | + |
| HSF2 | Heat shock Factor 2 | 0.974 | - |
| Hmx2/Nkx5-2 | Hmx2/Nkx5-2 homeodomain transcription factor | 0.933 | + |
| Pdx1 | Insulin promoter factor 1; pancreatic and duodenal homeobox 1 (Pdx1) | 0.924 | + |
| LMX1A | LIM homeobox transcription factor 1 alpha | 1.0 | + |
| NACA | Nascent polypeptide-associated complex subunit alpha 1 | 1.0 | - |
| Oct1 | Octamer binding factor 1 | 0.921 | + |
| POU6F1 | POU class 6 homeobox 1 | 0.973 | + |
| STAT 5B | Signal transducer and activator of transcription 5B | 0.973 | + |
| SIX 4 | Sine oculis homeobox homolog 4 | 0.96 | - |
| NMP4 | Nuclear matrix protein 4 | 0.971 | + |
| MSX | Homeodomain proteins MSX-1 and MSX-2 | 0.989 | - |
| AREB6 | Atp1a1 regulatory element binding factor 6 | 1.0 | - |
| VCR | Vertebrate caudal related homeodomain protein | 0.963 | + |
Protein Interactions
Most of the predicted protein interactions with C6orf10 are based solely on text mining and information gathered from genome-wide association studies. The two proteins with the highest interaction scores were Butyrophilin-like protein 2 (BTNL2) and Tetratricopeptide repeat domain containing TTC32.[22] BTNL2 is a negative regulator of T-cell activity and member of the immunoglobulin superfamily. BTNL2 is located in the C6orf10 gene neighborhood. TTC32 is from a protein family of structural repeat motifs that mediate protein-protein interactions in the formation of protein-protein complexes.[23] This may indicate the potential for C6orf10 to interact with another protein for form a complex.
Clinical significance
C6orf10 has been found to be associated with both neurodegenerative diseases and autoimmune diseases. These associations are mostly obtained from genome wide association studies. Common neurodegenerative diseases associated with C6orf10 include frontotemporal dementia, Parkinson's disease,[24] and Alzheimer's disease.[24] Autoimmune diseases associated with C6orf10 include Rheumatoid arthritis,[25][26][24][27] psoriasis,[28][27] multiple sclerosis,[29][24] Grave's disease[24] and lupus.
Homologs
Orthologs
By searching the NCBI BLAST database[30] for protein-protein interactions, it was found that C6orf10 is a protein only found in mammals. The BLAST database found the highest number of homologs in the Primates, Artiodactyla, and Carnivora. There were only a couple of homologs in the taxonomic orders of Rodentia, Chiroptera, and Perissodactyla. In the orders of Scandentia, Eulipotyphyla, Tubulidentata and sirenia there was only one complete homolog, but a few partial sequences do exist. There were partial protein sequences in Lagomorpha, Dermoptera, and Macroscelidea and there were no orthologs in Diprotodontia, Didelphimorphia, Cetacea, Dasyuromorphia, Pilosa, Monotremata, and Proboscidea. No homologs were found outside of mammals.
C6orf10 Isoform X4 Orthologs
| Latin Name | Common Name | Identity | Median Date of divergence (MYA) | |
| Primates | Homo sapiens | Human | 100% | 0 |
| Primates | Gorilla gorilla gorilla | Gorila | 83.36% | 8.61 |
| Primates | Pongo abelii | Sumatran Orangutan | 82.81% | 15.2 |
| Carnivora | Canis lupus familiaris | Dog | 49.79% | 94 |
| Carnivora | Canis lupus dingo | Australian Dog | 49.33% | 94 |
| Artiodactyla | Bubalus bubalis | Water Buffalo | 49.16% | 94 |
| Artiodactyla | Equus caballus | Horse | 49.00% | 94 |
| Artiodactyla | Odocoileus virginianus texanus | White Tailed Deer | 44.59% | 94 |
| Rodentia | Chinchilla lanigera | Chinchilla | 41.99% | 88 |
| Rodentia | Mus caroli | Ryuku mouse | 41.06% | 88 |
| Carnivora | Felis catus | Cat | 40.77% | 94 |
| Rodentia | Rattus norvegicus | Brown Rat | 37.45% | 88 |
Paralogs
C6orf10 has one paralog that diverged about 135.6 million years ago. This paralog is called Thioredoxin domain containing protein 2 (TXNDC2).[30]
[1] BLAST: Basic Local Alignment Search Tool. National Center for Biotechnology InformationAvailable at: https://blast.ncbi.nlm.nih.gov/Blast.cgi. Accessdate 4 March 2019.
References
- ENSG00000236672, ENSG00000204296, ENSG00000206310, ENSG00000234280, ENSG00000237881, ENSG00000232106, ENSG00000226892 GRCh38: Ensembl release 89: ENSG00000206245, ENSG00000236672, ENSG00000204296, ENSG00000206310, ENSG00000234280, ENSG00000237881, ENSG00000232106, ENSG00000226892 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Stammers M, Rowen L, Rhodes D, Trowsdale J, Beck S (April 2000). "BTL-II: a polymorphic locus with homology to the butyrophilin gene family, located at the border of the major histocompatibility complex class II and class III regions in human and mouse". Immunogenetics. 51 (4–5): 373–82. doi:10.1007/s002510050633. PMID 10803852.
- "Entrez Gene: C6orf10 chromosome 6 open reading frame 10".
- "AceView: Gene:C6orf10, a comprehensive annotation of human, mouse and worm genes with mRNAs or ESTsAceView". www.ncbi.nlm.nih.gov. Retrieved 25 February 2019.
- "TSBP1 testis expressed basic protein 1 [Homo sapiens (human)] - Gene - NCBI". www.ncbi.nlm.nih.gov. Retrieved 25 February 2019.
- "Tissue expression of C6orf10 - Summary - The Human Protein Atlas". www.proteinatlas.org. Retrieved 25 February 2019.
- "Clustal Omega < Multiple Sequence Alignment < EMBL-EBI". www.ebi.ac.uk. Retrieved 6 May 2019.
- "MView < Multiple Sequence Alignment < EMBL-EBI". www.ebi.ac.uk. Retrieved 6 May 2019.
- "The Mfold Web Server | mfold.rit.albany.edu". unafold.rna.albany.edu. Retrieved 5 May 2019.
- "SAPS < Sequence Statistics < EMBL-EBI". www.ebi.ac.uk. Retrieved 6 May 2019.
- "ExPASy - Compute pI/Mw tool". web.expasy.org. Retrieved 6 May 2019.
- "PSORT II Prediction". psort.hgc.jp. Retrieved 6 May 2019.
- "NCBI CDD CDD Conserved Protein Domain Neuromodulin_N". www.ncbi.nlm.nih.gov. Retrieved 6 May 2019.
- "Coils output". embnet.vital-it.ch. Retrieved 6 May 2019.
- "CFSSP: Chou & Fasman Secondary Structure Prediction Server". www.biogem.org. Retrieved 6 May 2019.
- npsa-prabi.ibcp.fr https://npsa-prabi.ibcp.fr/cgi-bin/secpred_sopma.pl. Retrieved 6 May 2019. Missing or empty
|title=(help) - "ProP 1.0 Server - prediction results". www.cbs.dtu.dk. Retrieved 6 May 2019.
- "TSBP1 testis expressed basic protein 1 [Homo sapiens (human)] - Gene - NCBI". www.ncbi.nlm.nih.gov. Retrieved 6 May 2019.
- "Genomatix - NGS Data Analysis & Personalized Medicine". www.genomatix.de. Retrieved 6 May 2019.
- "Human BLAT Search". genome.ucsc.edu. Retrieved 6 May 2019.
- "STRING: functional protein association networks". string-db.org. Retrieved 6 May 2019.
- Zeytuni N, Zarivach R (March 2012). "Structural and functional discussion of the tetra-trico-peptide repeat, a protein interaction module". Structure. 20 (3): 397–405. doi:10.1016/j.str.2012.01.006. PMID 22404999.
- Zhang M, Ferrari R, Tartaglia MC, Keith J, Surace EI, Wolf U, et al. (October 2018). "A C6orf10/LOC101929163 locus is associated with age of onset in C9orf72 carriers". Brain. 141 (10): 2895–2907. doi:10.1093/brain/awy238. PMC 6158742. PMID 30252044.
- Verma A, Basile AO, Bradford Y, Kuivaniemi H, Tromp G, Carey D, Gerhard GS, Crowe JE, Ritchie MD, Pendergrass SA (2016). "Phenome-Wide Association Study to Explore Relationships between Immune System Related Genetic Loci and Complex Traits and Diseases". PLOS ONE. 11 (8): e0160573. Bibcode:2016PLoSO..1160573V. doi:10.1371/journal.pone.0160573. PMC 4980020. PMID 27508393.
- Zheng W, Rao S (August 2015). "Knowledge-based analysis of genetic associations of rheumatoid arthritis to inform studies searching for pleiotropic genes: a literature review and network analysis". Arthritis Research & Therapy. 17: 202. doi:10.1186/s13075-015-0715-1. PMC 4529690. PMID 26253105.
- Malavia TA, Chaparala S, Wood J, Chowdari K, Prasad KM, McClain L, Jegga AG, Ganapathiraju MK, Nimgaonkar VL (2017). "Generating testable hypotheses for schizophrenia and rheumatoid arthritis pathogenesis by integrating epidemiological, genomic, and protein interaction data". NPJ Schizophrenia. 3: 11. doi:10.1038/s41537-017-0010-z. PMC 5441529. PMID 28560257.
- Feng BJ, Sun LD, Soltani-Arabshahi R, Bowcock AM, Nair RP, Stuart P, Elder JT, Schrodi SJ, Begovich AB, Abecasis GR, Zhang XJ, Callis-Duffin KP, Krueger GG, Goldgar DE (August 2009). "Multiple Loci within the major histocompatibility complex confer risk of psoriasis". PLoS Genetics. 5 (8): e1000606. doi:10.1371/journal.pgen.1000606. PMC 2718700. PMID 19680446.
- Lin X, Deng FY, Mo XB, Wu LF, Lei SF (January 2015). "Functional relevance for multiple sclerosis-associated genetic variants". Immunogenetics. 67 (1): 7–14. doi:10.1007/s00251-014-0803-4. PMID 25308886.
- "BLAST: Basic Local Alignment Search Tool". blast.ncbi.nlm.nih.gov. Retrieved 6 May 2019.
External links
- Human C6orf10 genome location and C6orf10 gene details page in the UCSC Genome Browser.
Further reading
- Ficarro S, Chertihin O, Westbrook VA, White F, Jayes F, Kalab P, Marto JA, Shabanowitz J, Herr JC, Hunt DF, Visconti PE (March 2003). "Phosphoproteome analysis of capacitated human sperm. Evidence of tyrosine phosphorylation of a kinase-anchoring protein 3 and valosin-containing protein/p97 during capacitation". The Journal of Biological Chemistry. 278 (13): 11579–89. doi:10.1074/jbc.M202325200. PMID 12509440.
- Suzuki Y, Yoshitomo-Nakagawa K, Maruyama K, Suyama A, Sugano S (October 1997). "Construction and characterization of a full length-enriched and a 5'-end-enriched cDNA library". Gene. 200 (1–2): 149–56. doi:10.1016/S0378-1119(97)00411-3. PMID 9373149.
- Maruyama K, Sugano S (January 1994). "Oligo-capping: a simple method to replace the cap structure of eukaryotic mRNAs with oligoribonucleotides". Gene. 138 (1–2): 171–4. doi:10.1016/0378-1119(94)90802-8. PMID 8125298.