Rufous fantail
The rufous fantail (Rhipidura rufifrons) is a small Passerine bird,[2] most commonly known also as the black-breasted rufous-fantail or rufous-fronted fantail, which can be found in Australia, Indonesia, Micronesia, New Guinea and the Solomon Islands.[3] In these countries they inhabit rainforests, wet forests, swamp woodlands and mangroves.[4]
| Rufous fantail | |
|---|---|
 | |
| Adult | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Aves |
| Order: | Passeriformes |
| Family: | Rhipiduridae |
| Genus: | Rhipidura |
| Species: | R. rufifrons |
| Binomial name | |
| Rhipidura rufifrons (Latham, 1801) | |
 | |
| = Extant (resident) = Extant (non breeding) | |
Characteristic of species that have a large range, the rufous fantail has many subspecies. However the taxonomic treatment of its subspecies and other relatives is still debated.[5] The rufous fantail is easily distinguished by their orange-reddish-brown back, rump and base of tail.[6] They have a black and white breast that grades into a white colour on the chin and throat.
They are migratory, travelling to south-eastern Australia in the spring to breed,[7] and then north in the autumn.[8]
The rufous fantail tends to feed on small insects in the lower parts of the canopy. They are very active birds making short, frequent flights. They may also hop between foliage or on the ground, during foraging.[6]
Although their population is thought to be declining, their relatively large range and abundance make them a species of least concern according to the IUCN.[1]
Taxonomy
The rufous fantail has complex evolutionary relationships and sometimes this results in conflicting taxonomy. This is not uncommon since taxonomies are merely hypotheses of a species' evolutionary status. Debate is still currently ongoing about the taxonomic treatment of the rufous fantail's subspecies and its related species.[5]
History of naming
The rufous fantail was first described by Latham in his 1801 work, Index Ornithologicus initially as Muscicapa ruffifrons.[9] It was later reclassified into the genus Rhipidura by Vigors and Horsfield.[10] The rufous fantail's scientific name is consequently Rhipidura ruffifrons. Rhipidura is derived from Greek: ρϊπός (pronounced rhipido), meaning fan-like and οὐρά (pronounced oura), meaning tail. Ruffifrons comes from two Latin words: rufus meaning red and frons meaning the forehead.[11]
Alternate names
The rufous fantail is also known by numerous other English names as well as several names in different languages. Some common English names include: rufous-fronted fantail, wood fantail, rufous-fronted flycatcher, wood flycatcher, red fantail, allied flycatcher, rufous flycatcher, rufous fan, red fan or redstart.[11]
Related species
It is one of over 40 member species of the genus Rhipidura, commonly known as the fantails. Within the genus it belongs to a group of five closely related species: R. rufidorsa, R. brachyrhyncha, R. dahli, R. teysmanni and R. dryas. A molecular phylogeny study showed the Arafura fantail (Rhipidura dryas) to be its closest relative.[12]
It forms a superspecies with R. dryas and R. semirubra, and all three are often considered conspecific. All are part of a larger species group that also includes R. teysmanni, R. superflua, R. dedemi, R. opistherythra, R. lepida, R. rufidorsa, R. dahli, R. matthiae and R. malaitae.[13]
Evolution
| ||||||||||||||||||||||||||||||
| Consensus phylogeny tree of a clade within the fantails (Rhipiduridae) according to Nyári et al. (2009)[12] |
The current spatial distribution suggests an ancestry originating in the Papuan region, most likely New Guinea.[13]
The ancestral form may have had a white chin, white throat, and a light grey breast as well as a greyish-brown head and back.[13] Indirect evidence suggests that ancestral species undertook two periods of aggressive range expansions (dispersal) separated by a period of inactivity.[13]
During the former dispersal period, it is hypothesised by Mayr et al. (1946)[13]
that:
- Dispersal north and westwards formed the superflua on Buru, teijsmanni on Celebes, and lepida on Palau.
- Dispersal to Tenimber Islands in the Banda Sea formed the opistherythra.
- Dispersal to Northern New Guinea formed the rufidorsa.
- Dispersal to Bismarck Archipelago formed the dahli-antonii-matthiae series.
- Dispersal to Southeastern New Guinea and nearby islands evolved into the true rufifrons subspecies.
During the latter dispersal period, the true rufifrons group underwent "explosive sub-speciation". This is in stark contrast to the other members whose evolution was stagnant. The true rufifrons further evolved into eighteen subgroups.[13]
Subspecies
The rufous fantail is a superspecies comprising eighteen recognised subspecies.[14][15] In alphabetical order, these are:
| Subspecies | Identified | Range | Notes |
|---|---|---|---|
| R. f. agilis | Mayr, 1931 | Santa Cruz Group of islands (Solomon Islands) | It has a dull brown forehead,[16] black throat and a white stripe across the chin.[17] It lacks a supercilium.[17] |
| R. f. brunnea | Mayr, 1931 | Malaita (Solomon Islands) | It is similar to R. f. rufofronta but its upperparts and ear-coverts are darker.[17] |
| R. f. commoda | E. J. O. Hartert, 1918 | Within the Solomon Islands, they are found in the Bougainville Island of Papua New Guinea, the Guadacanal Island, Malaita Island, New Georgia group of islands and the Makira group of islands.[16] | It has a brown head, mantle and wings, bright orange-rufous rump, tail-base and forehead, and remainder of tail black with broad white tips. White throat, black breast-band, black spots on lower breast, and buffy underparts. Forest, especially open forest. Understorey. Hyperactive, with drooped wings and waving fanned tail. Common.[18] Half its central tail feathers are reddish brown.[17] |
| R. f. granti | E. J. O. Hartert, 1918 | Central Solomon Islands | It is like R. f. commoda apart from having black ear-coverts instead.[17] |
| R. f. intermedia | North, 1902 | E Queensland (Cooktown to NSW border); > to north | It has blacker patches on its lores and below eye than R.f. nominate.[6] |
| R. f. kuperi | Mayr, 1931 | Santa Anna (Solomon Islands) | It is otherwise identical to R.f. russata but it has more reddish-brown colouration on its forehead and a darker neck which blends into a reddish-brown mantle.[17] |
| R. f. louisiadensis | E. J. O. Hartert, 1899 | D'Entrecasteaux and Louisiade archipelagos | |
| R. f. mariae | R. H. Baker, 1946 | Agiguan and Rota (Mariana Islands) | |
| R. f. melaenolaema | Sharpe, 1879 | Vanikoro (Santa Cruz Islands) | It is similar to R. f. utupuae but has a less white on its short supercilium[17] and olive-grey upperparts.[16] |
| R. f. rufifrons | Latham, 1801 | SE Australia (ne NSW to s and cent. Victoria); > to north | The tips of its tail are a light brownish-grey colour.[6] |
| R. f. rufofronta | E. P. Ramsay, 1879 | Guadalcanal (Solomon Islands) | It is otherwise identical to R. f. commoda but it has blackish-brown ear-coverts and only the bottom tail feathers are reddish-brown in colour.[17] |
| R. f. russata | Tristram, 1879 | San Cristóbal (Solomon Islands) | It is identical to R. f. commoda but has bright orange mantle instead of brown[19] and a narrow black breast-band.[17] |
| R. f. saipanensis | E. J. O. Hartert, 1898 | Saipan and Tinian (Mariana Islands) | |
| R. f. torrida | Wallace, 1865 | Halmahera, Ternate, Bacan and Obi Maluk Islands |  Diagram of R. f. torrida. |
| R. f. ugiensis | Mayr, 1931 | Ugi (Solomon Islands) | It is similar to R.f. commoda apart from mostly or completely lacking the white on the throat.[19] Also, its upperparts are a relatively lighter reddish brown.[17] |
| R. f. uraniae | E. J. O. Hartert, 1899 | Guam (Mariana Islands) | Extinct.[17] |
| R. f. utupuae | Mayr, 1931 | Utupua (Solomon Islands) | It has a white forehead and supercilium, a rusty-brown crown and mantle, black throat and chin.[16] |
| R. f. versicolor | Hartlaub & Finsch, 1872 | Yap (Caroline Islands) |
Description

Adults are medium-sized birds, generally ranging from 14.5 cm – 18.5 cm in length, averaging at around 15 cm;[20] their wingspan is between 18 cm – 22.5 cm, averaging at around 21 cm.[6] They weigh roughly 10 grams.[6] The male and female of the species look identical. However, females are generally smaller than the males.[10]
The forehead is a richly reddish-brown colour across the eyes. The eyes have a white arc just below them. The top of the head, back of the neck and the upper back, transition from an olive to reddish-brown colour, which then blends into a blackish-brown, long, fan-shaped tail. This blackish-brown tail, contrasts with the base of the tail, which is tipped with a paler colour, often white.[16]
It has black ear-coverts (feathers over the ears, just below and behind the eyes). The throat is white (in most subspecies), and there is a black bar across the upper breast. Below this, the lower breast is off-white with black scale-like spots[21] which transitions into an off-white colour towards the centre of the abdomen. The eyes, bill and feet of the bird are all a brown colour.[10]
The aforementioned colours do not change during different seasons.[6] However, compared to the adults, the juveniles have generally duller coloured backs and marginally browner tails and underparts.[16] On the other hand, the base of the bill and their legs are a paler brown relative to an adult's.[6]
A physical description that may help distinguishing between the different subspecies can be found in the subspecies section of this article.
The plumage in the immature birds is similar to those of the adults and in both sexes.[6] Adults moult annually prior to the breeding season, and this basic plumage does not vary.[22]
Vocalisations
These have been not been well characterised in the rufous fantail.[23] Nevertheless, it has been observed to create several different types of sounds such as chips, buzzes, and scolds.[24] Their "chip call" is often what first attracts an observer's attention.[6] This call is high pitched, with two chip noises given in quick succession. It is produced during food searching, territory defence and can be used as an alarm call when a predator is identified.[25] They sing after sunset from perches, one reason is to attract the opposite sex.[23]
Similar species
Whilst it is similar in size and shape to grey fantails (Rhipidura albiscapa), it has a slightly larger fantail and creates higher pitched and softer songs.[6]
Its diagnostic physical features: orange-reddish-brown back, rump and base of tail – easily differentiate it from other fantails.[6] Moreover, it can be further distinguished from similar fantails as it tends to forage in shady and moist regions of habitats that are close to the ground.[6]
Distribution and habitat
Distribution
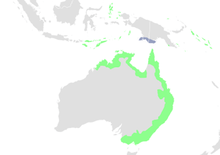
The rufous fantail can be found in parts of Australia, southeast Asia, and in the Oceanic regions of Micronesia and Melanesia.[3] They are residents of the Lesser Sunda Islands and the Maluku Islands of Indonesia, southern New Guinea and its associated islands, the Solomon Islands, the Marianas and the Carolinian island of Yap. In Australia, they are found in the northern and eastern coastal regions.[26]
Certain subspecies tend to be restricted to some ranges. See the subspecies section of this article for more detailed information.
Habitat
The rufous fantail inhabits moist and moderately dense habitats.[6][27] Within these areas, it has astonishingly large variations in habitat requirements.[13] They can be found in eucalyptus forests, mangroves,[4] rainforests and woodlands (usually near a river or swamp). Rarely, they have even been found in dry sclerophyll forests.[6] Apart from open grasslands and open arid areas, there are not many major types of landscape in the Australo-Pauan region that cannot be inhabited by at least one subspecies of the rufous fantail.[13]
Rufous fantails will generally occupy the lower levels of their habitat, the understorey or the subcanopy, straying no further than 6m from the ground.[2] Different subspecies may tend to prefer slightly different habitats which can be sometimes discrete or overlapping.[13]
Behaviour and ecology
Studies on rufous fantail social behaviour are sparse.[25] Some observers have anecdotally described them as curious and trustful,[21] whilst others depict them as shy creatures.[27] However, there is consensus in that they are almost always portrayed as hyperactive, constantly on the move, fidgeting and waving a fanned-out tail.[6][21]
They are usually observed flitting about in the lower layers of their habitat, in close association with the shade, making short, frequent flights separated by brief moments of perching and sometimes hopping between foliage or onto the ground.[6]
Breeding
When they are spotted, they are usually either on their own or in pairs. Although their social bonding is not well known, they nest in pairs and are thought to be monogamous.[25] Males will produce vocal songs to both defend and advertise their territory.[25] Sometimes this can result in intense, rapid and prolonged vocal "battles".[25]
After pairing, both will search for a suitable breeding site. The female has the final say on nest location.[23] Some males have been known to feed their paired females for up to 2–3 weeks before and during the selection of the nest site and building of the nest.[25] Their breeding sites are mainly in rainforest regions or sheltered, humid gulies[7] with an abundance of dense cover such as trees, saplings, shrubs and vines.[23] The nest, will usually be built at the fork between two nearly horizontal tree branches in proximity to a water source, such as a stream. The structure of the nest is often compared to a wine glass with a broken bottom stand.[21] The nest is built, usually in November, December and January,[21] using thin strips of tree bark, grass, moss rootlets and decayed wood.[22]
The eggs themselves are round or oval in shape and occasionally have a point at one end.[22] Their colour is generally described as somewhere between a pale cream to yellowish white.[22] These have light brown and purplish markings or spots.[10][21] There are normally 3 or 4 eggs produced in a nest.[22]
Both the males and the females give (frequently alternating) parental care,[22] which includes: feeding their nestlings and removing their feacal sacs from the nest.[25] Whilst only females have actually been observed to incubate (brood) laid eggs, it is assumed males can do this as well.[25]
Four to five weeks after hatching, the young will leave the nest. However, they will remain near their nest (natal area) until they undertake their first migration.[22]
Migration
Some subspecies have slightly differing migration patterns. However, the vast majority exhibit strong migratory behavior – they use the same route year after year and have regular departure and arrival times.[23] They migrate to south-eastern Australia in the spring to breed, beginning in September, peaking in October,[7] and then north in the autumn during March and April.[8] This has been well characterised.[28]
Food and feeding

They eat mostly small insects[29] and will often join mixed species feeding flocks to do so.[25] These usually comprise other small Passerine birds[2] such as: the spectacled monarch, the little shrikethrush, the large-billed scrubwren and less occasionally, the green-backed honeyeater.[30]
The rufous fantail is mostly an aerial forager, rarely perching during feeding.[2] Prey are found during almost continuous movement in and between vegetation. They stop (perch) for very short periods of time, during which they fan their tails.[31] Much more rarely, they perch for longer than five seconds to survey surroundings.[30]
Once a prey is located, they will pursue it by exhibiting extremely agile and maneuverabile flight within the canopy ( by salling, flush-pursuit or flutter-chase).[30]
However, they are versatile foragers,[2] also capable of different foraging methods, occasionally hovering to glean prey from leaves and (very rarely) from the ground and other fallen debris.[2][29] They have longer legs relative to other Rhipidura species, enabling them to have agile movement on the ground as well.[10]
Threats/survival
Many eggs and young are lost to the suspected predator, the pied currawong (Strepera graculina).[21]
Relationship to humans

The logging of forests has been shown to influence foraging preference, changing from the forest floor to the lower canopy. However, they prefer undisturbed forests.[26] Logging decreases breeding habitat and increases the risk of fragmentation, particularly if these forests are in migration routes.[26]
Status
The range of the rufous fantail is very extensive. On this basis it does not have a range small enough to be considered vulnerable (<20,000 km2). Although the population size has not been properly characterised, it is thought to be declining, but not rapidly enough to be placed into vulnerable status. Therefore, the species is of least concern as classified by the IUCN.[1]
References
- BirdLife International (2013). "Rhipidura rufifrons". IUCN Red List of Threatened Species. 2013. Retrieved 26 November 2013.CS1 maint: ref=harv (link)
- Craig, Robert J.; Beal, Kathleen G. (2001). "Microhabitat partitioning among small passerines in a Pacific island bird community". The Wilson Bulletin. 113 (3): 317–326. doi:10.1676/0043-5643(2001)113[0317:MPASPI]2.0.CO;2.
- BirdLife International; Natureserve (2009). "TBird species distribution maps of the world". Retrieved 21 March 2012.
- Kutt A.S. (2007). "Bird assemblage in a dune-mangrove mosaic, Cairns, Queensland" (PDF). Australian Zoologist. 34 (2): 158–164. doi:10.7882/az.2007.013. Archived from the original (PDF) on 21 March 2012. Retrieved 16 March 2012.
- Christidis, Les; Boles, Walter (2008). Systematics and Taxonomy of Australian Birds. Csiro Publishing. p. 197. ISBN 978-0-643-06511-6. Retrieved 20 March 2012.
- Higgins et al. (HANZAB) p.161
- Hindwood (1948). "The Rufous Fantail in City Buildings". Emu. 48: 31–32. doi:10.1071/MU948031.
- Campbell, Bruce; Lack, Elizabeth (1985). A Dictionary of Birds. Harrell Books. p. 206. ISBN 978-0931130120. Retrieved 17 March 2012.
- Latham, John (1801). Supplementum indicis ornithologici sive systematis ornithologiae (in Latin). London: Leigh & Sotheby. p. l.
- Gould, John (1865). Handbook to The birds of Australia, Volume 1. London; J. Gould. pp. 240–242. Retrieved 17 March 2012.
- Higgins et al. (HANZAB) p.160
- Nyári, Árpád S; Benz, Brett W.; Jønsson, Knud A.; Fjeldså, Jon; Moyle, Robert G. (2009). "Phylogenetic relationships of fantails (Aves: Rhipiduridae)". Zoologica Scripta. 38 (6): 553–561. doi:10.1111/j.1463-6409.2009.00397.x.
- Mayr, Erynst; Moynihan, Martin (1946). "Evolution in the Rhipidura ruffifrons group". American Museum Novitates. 1321: 1–21. hdl:2246/4445.
- Matyr, Ernst; Diamond, Jared (2001). The birds of northern Melanesia: speciation, ecology & biogeography. Oxford University Press. p. 176. ISBN 978-0195141702. Retrieved 17 March 2012.
- Clements, J.F.; Schulenberg, T.S.; Iliff, M.J.; Sullivan, B.L.; Wood, C.L.; Roberson, D. (Aug 2011). "The Clements checklist of birds of the world: Version 6.6". Retrieved 18 March 2012.
- Dutson, Guy (Birds of Melanesia) p. 202
- Dutson, Guy (Birds of Melanesia) p. 382
- Dutson, Guy (Birds of Melanesia) p. 178
- Dutson, Guy (Birds of Melanesia) p. 192
- Dutson, Guy (Birds of Melanesia) p. 381
- Chaffer, N. (1929). "The Rufous Fantail in the National Park" (PDF). Emu. 29: 48–49. doi:10.1071/MU929048. Retrieved 18 March 2012.
- Higgins et al. (HANZAB) p.169
- Higgins et al. (HANZAB) p.168
- Ramsey (1987). "Covariate Adjustments to Effective Area in Variable-Area Wildlife Surveys". Biometrics. 43 (1): 1–11. doi:10.2307/2531943. JSTOR 2531943.
- Higgins et al. (HANZAB) p.167
- Higgins et al. (HANZAB) p.163
- Thomas, Richard; Thomas, Sarah; Andrew, David; McBride, Alan (1996). The Complete Guide to Finding the Birds of Australia (2nd ed.). Frogmouth Publications. p. 368. ISBN 978-0952806509. Retrieved 17 March 2012.
- Higgins et al. (HANZAB) p.164
- Higgins et al. (HANZAB) p.165
- Higgins et al. (HANZAB) p.166
- Jackson, Janey; Elgar, Mark (1993). "The Foraging Behaviour of the Willie Wagtail Rhipidura leucophrys: Why Does it Wag its Tail?". Emu. 93 (4): 286. doi:10.1071/MU9930284.
Cited texts
- Dutson, Guy (2011). Birds of Melanesia: Bismarcks, Solomons, Vanuatu and New Caledonia. Bloomsbury Publishing Plc. ISBN 978-0713665406.
- Higgins, P.J; Peter, J.M; Cowling, S.J (2006). Handbook of Australian, New Zealand and Antarctic Birds: Boatbill to Starlings. 7. Melbourne, Australia: Oxford University Press. ISBN 978-0195539967.
External links
| Wikimedia Commons has media related to Rhipidura rufifrons. |
| Wikispecies has information related to Rhipidura rufifrons |
- Photos, videos and sounds – Internet Bird Collection