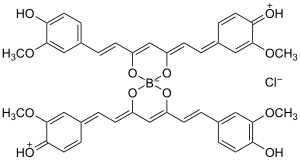
Rosocyanine
Rosocyanine and rubrocurcumin are two red colored materials, which are formed by the reaction between curcumin and borates.
 | |
| Names | |
|---|---|
| Other names
Rosocyanine | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| [B(C21H19O6)2]Cl (as chloride) | |
| Molar mass | 781.013 g/mol |
| Appearance | dark-green colored solid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Application
The color reaction between borates and curcumin is used within the spectrophotometrical determination and quantification of boron present in food or materials. Curcumin is a yellow coloring natural pigment found in the root stocks of some Curcuma species, especially Curcuma longa (turmeric), in concentrations up to 3%. In the so-called curcumin method for boron quantification it serves as reaction partner for boric acid. The reaction is very sensitive and so the smallest quantities of boron can be detected. The maximum absorbance at 540 nm for rosocyanine is used in this colorimetric method. The formation of rosocyanine depends on the reaction conditions. The reaction is carried out preferentially in acidic solutions containing hydrochloric or sulfuric acid. The color reaction also takes place under different conditions; however, in alkaline solution, gradual decomposition is observed. The reaction might be disturbed at higher pH values, interfering with other compounds.
Rosocyanine is formed as a 2:1 complex from curcumin and boric acid in acidic solutions. The boron complexes formed with rosocyanine are dioxaborines (here a 1,3,2-dioxaborine). Curcumin possesses a 1,3-diketone structure and can therefore be considered as a chelating agent. Unlike the simpler 1,3-diketone–containing compound acetylacetone (which forms acetylacetonate complexes with metals), the entire skeleton of curcumin is in resonance with the 1,3-dicarbonyl section, making the backbone an extended conjugated system. Investigations of the structure have shown that the positive charge is distributed throughout the molecule. In rosocyanine, the two curcumin moieties are not coplanar but rather perpendicular relative to one another (as seen in the 3D model), as a result of the tetrahedral geometry of tetracoordinate boron. The same applies to rubrocurcumin.
In order to exclude the presence of other materials during the boron quantification using the curcumin method, a variant of the process was developed. In this process, 2,2-dimethyl-1,3-hexanediol or 2-ethyl-1,3-hexanediol are added, in addition to curcumin, to a neutral solution of the boron-containing solution. The complex formed between boron and the 1,3-hexanediol derivative is removed from the aqueous solution by extraction in an organic solvent. Acidification of the organic phase yields rubrocyanine, which can be detected by colorimetric methods. The reaction of curcumin with borates in presence of oxalic acid produces the coloring compound rubrocurcumin.
Characteristics
Rosocyanine is a dark green solid with a glossy, metallic shine that forms red colored solutions. It is almost insoluble in water and some organic solvents, very slightly soluble (up to 0.01%) in ethanol, and somewhat soluble (approximately 1%) in pyridine, sulfuric acid, and acetic acid. An alcoholic solution of rosocyanine temporarily turns deeply blue on treatment with alkali.
In rubrocurcumin one molecule of curcumin is replaced with oxalic acid. Rubrocurcumin produces a similar red colored solution. Rosocyanine is an ionic compound, while rubrocurcumin is a neutral complex.
See also
References
- Schlumberger, M. E. (1866). "Sur la réaction de l'acide borique sur la curcumine" [On the reaction of boric acid upon curcumin]. Bulletin de la Société Chimique de Paris. 5 (1): 194–202. ISSN 0991-6504.
- Clarke, L.; Jackson, C. L. (1908). "Rosocyanine". American Chemical Journal. 39: 696–719. ISSN 0096-4085. LCCN 14006052. CODEN: ACJOAZ.
- Spicer, G. S.; Strickland, J. D. H. (1952). "Compounds of Curcumin and Boric acid. Part I. The Structure of Rosocyanin". Journal of the Chemical Society. 1952 (article 906): 4644–4650. doi:10.1039/JR9520004644. ISSN 0368-1769. CODEN: JCSOA9.
- Bellamy, L. J.; Spicer, G. S.; Strickland, J. D. H. (1952). "Compounds of Curcumin and Boric acid. Part III. Infra-red Studies of Rosocyanine and Allied Compounds". Journal of the Chemical Society. 1952 (article 908): 4653–4656. doi:10.1039/JR9520004653. ISSN 0368-1769. CODEN: JCSOA9.
- Spicer, G. S.; Strickland, J. D. H. (1958). "Determination of microgram and submicrogram amounts of boron. I. Absorptiometric determination with curcumin". Analytica Chimica Acta. 18: 231–239. doi:10.1016/S0003-2670(00)87133-0. ISSN 0003-2670. CODEN: ACACAM.
- Roth, H. J.; Miller, B. (1964). "Zur Kenntnis der Farbreaktion zwischen Borsäure und Curcumin. I. Borinsäure-Curcumin-Komplexe" [On the understanding of the dye reaction between boric acid and curcumin. I. Borinate–curcumin complexes]. Archiv der Pharmazie. 297 (10): 617–623. doi:10.1002/ardp.19642971007. ISSN 0376-0367. CODEN: APBDAJ.
- Roth, H. J.; Miller, B. (1964). "Zur Kenntnis der Farbreaktion zwischen Borsäure und Curcumin. II. Zur Konstitution des Rosocyanins und Rubrocurcumins" [On the understanding of the dye reaction between boric acid and curcumin. II. On the structure of rosocyanine and rubrocurcumin]. Archiv der Pharmazie. 297 (11): 660–673. doi:10.1002/ardp.19642971104. ISSN 0376-0367. CODEN: APBDAJ.
- Umland, F.; Thierig, D.; Mueller, G. (1966). "Photometrische Bestimmung von Bor im Picogram-Bereich" [Photometric determination of boron in the picogram region]. Fresenius' Journal of Analytical Chemistry. 215 (5): 401–406. doi:10.1007/BF00510442. ISSN 0937-0633. CODEN: FJACEP.
- Quint, P.; Umland, F. (1979). "Über die Zusammensetzung des (1:2)-Bor-Curcumin-Chelates »Rosocyanin«" [On the composition of the (1:2) boron–curcumin chelate "rosocyanine"]. Fresenius' Journal of Analytical Chemistry. 295 (4): 269–270. doi:10.1007/BF00481491. ISSN 0937-0633. CODEN: FJACEP.
- Dyrssen, D. W.; Novikov, Yu. P.; Uppstrom, L. R. (1972). "Studies on the chemistry of the determination of boron with curcumin". Analytica Chimica Acta. 60 (1): 139–151. doi:10.1016/S0003-2670(01)81893-6. ISSN 0003-2670. CODEN: ACACAM.
- Kowalenko, C. G.; Lavkulich, L. M. (1976). "A modified curcumin method for boron analysis of soil extracts" (pdf). Canadian Journal of Soil Science. 56 (4): 537–539. doi:10.4141/cjss76-068. ISSN 0008-4271. CODEN: CJSSAR.
- Chevallerie-Haaf, U.; Meyer, A.; Henze, G. (1986). "Photometrische Bestimmung von Bor im Grund- and Oberflächenwasser" [Photometric determination of boron in ground and surface water]. Fresenius' Journal of Analytical Chemistry. 323 (3): 266–270. doi:10.1007/BF00464089. ISSN 0937-0633. CODEN: FJACEP.
- Donaldson, E. M. (1981). "Spectrophotometric determination of boron in iron and steel with curcumin after separation by 2-ethyl-1,3-hexanediol-chloroform extraction". Talanta. 28 (11): 825–831. doi:10.1016/0039-9140(81)80024-0. ISSN 0039-9140. PMID 18963014. CODEN: TLNTA2.
- Wikner, B. (1981). "Boron determination in natural waters with curcumin using 2,2-dimethyl-1,3-hexanediol to eliminate interferences". Communications in Soil Science and Plant Analysis. 12 (7): 697–709. doi:10.1080/00103628109367185. ISSN 0010-3624. CODEN: CSOSA2.