Rubrocurcumin
Rubrocurcumin is a red-colored dye that is formed by the reaction of curcumin and borates.
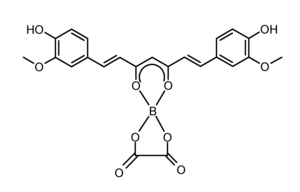 | |
| Names | |
|---|---|
| Other names
Rubrocurcumin | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C23H19BO10 | |
| Molar mass | 466.19 g/mol |
| Appearance | red solid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Synthesis
The reaction of curcumin with borates in presence of oxalic acid produces rubrocurcumin.[1]
Characteristics
Rubrocurcumin produces a red colored solution.
Rubrocurcumin is a neutrally charged composition, while rosocyanine is produced from ions. In rubrocurcumin, one molecule of curcumin is replaced with oxalate compared to rosocyanine.
Complexes with boron such as rubrocurcumin are called 1,3,2-dioxaborines.[1]
gollark: ddg! Price of bread per bread
gollark: What the potating OS? This says it's on Mac OS X 10.11 using Firefox 47?
gollark: Hey, I think I found you in the logs!
gollark: That is not valid English.
gollark: Why not?
References
- Rohde, D. (2002). Darstellung und Eigenschaftsuntersuchungen an 1,3,2-Dioxaborinen mit variablen Coliganden am Boratom [Presentation and characterization of 1,3,2-dioxaborins with variable coligands on the boron atom] (Dissertation). University Halle.
Further reading
- Spicer, G. S.; Strickland, J. D. H. (1952). "Compounds of Curcumin and Boric Acid. Part II. The Structure of Rubrocurcumin". Journal of the Chemical Society. London. 1952 (art. 907): 4650–4653. doi:10.1039/JR9520004650.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.