Rhizophydiales
Rhizophydiales are an important group of chytrid fungi. They are found in soil as well as marine and fresh water habitats where they function as parasites and decomposers.
| Rhizophydiales | |
|---|---|
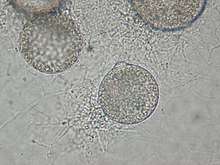 | |
| A member of Rhizophydiales growing on nutrient agar. Note the discharge papillae near the 12 o'clock position. | |
| Scientific classification | |
| Kingdom: | Fungi |
| Division: | Chytridiomycota |
| Class: | Chytridiomycetes |
| Order: | Rhizophydiales Letcher 2006[1] |
| Type species | |
| Rhizophydium globosum Schenk 1858 | |
Role in the environment

Rhizophydiales are parasites of a range of organisms, including invertebrates, other chytrids and algae, and they may have a role in natural control of aquatic populations, especially phytoplankton.[2][3] One member, Rhizophydium graminis, is a parasite of wheat roots, but causes no extensive damage to the plant. The only documented cases of a chytrid parasitizing vertebrates are Batrachochytrium dendrobatidis and Batrachochytrium salamandrivorans, members of this order. They are highly destructive pathogens of frogs and salamanders respectively.[4]
The majority of the described saprotrophic species of this order are biodegraders of pollen, with only a few growing on keratin, chitin, and cellulose. The transformational role of the Rhizophydiales in aquatic food webs is little studied but recently more recognized.[5]
Life history
Their thalli (=bodies) consist of two parts: an absorptive branching rhizoidal system that contains no nuclei and a multinucleate sporangium that ranges in shape from spherical, to oval, to pear-shaped, and to multi-lobed. The rhizoids attach the thallus to a substrate (food source) and absorbs nutrients. When the thallus is fully grown, the sporangium releases numerous, unwalled, uninucleate-zoospores, each bearing a single posteriorly directed flagellum.
The zoospore has to use its own stored food reserves (lipids and glycogen) as it swims until it attaches to a suitable host or substrate, absorbs its flagellum, produces a wall around itself, grows a germ tube that penetrates the substrate, and develops into a new thallus.[6] Zoospores of parasitic chytrids use light and chemical cues to locate hosts. Zoospores of Rhizophydium littoreum, a parasite of marine green algae, are positively phototactic toward blue light, a mechanism that might assure that zoospores swim to the photic zone where its host resides.[7] Zoospores of both R. littoreum and B. dendrobatidis exhibit chemotaxis to specific sugars, proteins and amino acids, also a mechanism by which zoospores might detect signals to potential hosts.[8][9]
Sexual reproduction is more rarely reported and occurs when two adjacent sporangia function as gametangia with one transferring all of its cytoplasmic contents into the other, resulting in the development of a thick-walled, lipid-laden resting spore.[10]
Phylogeny
Based on the work of "The Mycota: A Comprehensive Treatise on Fungi as Experimental Systems for Basic and Applied Research",[11] Powell and Letcher 2015[12]
| Rhizophydiales |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Taxonomic classification
The Rhizophydiales is an order of fungi that includes the following families and genera:
- Aquamycetaceae Letcher 2008
- Aquamyces Letcher 2008
- Alphamycetaceae Letcher 2008
- Angulomycetaceae Letcher 2008
- Angulomyces Letcher 2008
- Batrachochytriaceae Doweld 2013
- Batrachochytrium Longcore, Pessier & D.K. Nichols 1999[4] (no clear relatives)[15]
- Homolaphlyctis Longcore, Letcher & T.Y. James 2011 [16]
- Coralloidiomycetaceae Doweld 2014
- Coralloidiomyces Letcher 2008[17]
- Dinomycetaceae Karpov & Guillou 2014
- Dinomyces Karpov & Guillou 2014[18]
- Globomycetaceae Letcher 2008
- Globomyces Letcher 2008
- Urceomyces Letcher 2008
- Gorgonomycetaceae Letcher 2008
- Gorgonomyces Letcher 2008
- Halomycetaceae Letcher & Powell 2015
- Kappamycetaceae Letcher 2006
- Kappamyces Letcher & M.J. Powell 2005[20]
- Operculomycetaceae Doweld 2014
- Operculomyces M.J.Powell, Letcher & Longcore 2011[21]
- Pateramycetaceae Letcher 2008
- Pateramyces Letcher 2008
- Protrudomycetaceae Letcher 2008
- Protrudomyces Letcher 2008
- Rhizophydiaceae Werderm. 1954 [Tylochytriaceae Doweld 2014]
- Rhizophydium Schenk 1858
- Staurastromycetaceae Van den Wyngaert et al. 2017
- Staurastromyces Van den Wyngaert et al. 2017
- Terramycetaceae Letcher 2006
- Boothiomyces Letcher 2006
- Terramyces Letcher 2006[1]
- Uebelmesseromycetaceae Powell & Letcher 2015
- Uebelmesseromyces Powell & Letcher 2015[22]
Biodiversity
New species and genera are still being discovered in this order. A member of this order, Kappamyces, was the first phylogenetic genus of a chytrid circumscribed based primarily on monophyly demonstrated in molecular sequence analysis and confirmed with unique zoospore structure [20] Coralloidiomyces digitatus defied the original view held that the thallus of members of the Rhizophydiales was conservative. Collected from submersed mud at the edge of an oligotrophic lake in southern Argentina near the Andes in Patagonia, C. digitatus has a thallus with a sporangium shaped like a coral.[17]
References
- Letcher, P.M.; Powell, MJ; Churchill, PF; Chambers, JG (2006). "Ultrastructural and molecular phylogenetic delineation of a new order, the Rhizophydiales (Chytridiomycota)". Mycol. Res. 110 (Pt 8): 898–915. doi:10.1016/j.mycres.2006.06.011. PMID 16919432.
- Powell, M.J. (1993). "Looking at mycology with a Janus face. A glimpse at Chytridiomycetes active in the environment". Mycologia. 85 (1): 1–20. doi:10.2307/3760471. JSTOR 3760471.
- Ibelings, B.W.; et al. (2004). "Host parasite interactions between freshwater phytoplankton and chytrid fungi (Chytridiomycota)". J. Phycol. 40 (3): 437–453. doi:10.1111/j.1529-8817.2004.03117.x.
- Longcore, J.E..; Pessier, A.P.; Nichols, D.K (1999). "Batrachochytrium dendrobatidis gen. et sp. nov., a chytrid pathogenic to amphibians". Mycologia. 91 (2): 219–227. doi:10.2307/3761366. JSTOR 3761366.
- Kagami, M.; de Bruin, A; Ibelings, B.W.; Van Donk, E. (2007). "Parasitic chytrids: their effects on phytoplankton communities and food-web dynamics". Hydrobiologia. 578: 113–129. doi:10.1007/s10750-006-0438-z.
- Powell, M.J. (1976). "Ultrastructure and isolation of glyoxysomes (microbodies) in zoospores of the fungus Entophlyctis sp". Protoplasma. 89 (1–2): 1–27. doi:10.1007/BF01279325.
- Muehlstein, L.K.; Amon, J.P.; Leffler, D.L. (1987). "Phototaxis in the marine fungus Rhizopohydium littoreum". Appl. Environ. Microbiol. 53 (8): 1819–1821. PMC 204006. PMID 16347407.
- Muehlstein, L.K.; Amon, J.P.; Leffler, D.L. (1988). "Chemotaxis in the marine fungus Rhizophydium littoreum". Appl. Environ. Microbiol. 54 (7): 1668–1672. PMC 202725. PMID 16347677.
- Moss, A.S.; et al. (2008). "Chemotaxis of the amphibian pathogen Batrachochytrium dendrobatidis and its response to a variety of attractants". Mycologia. 100 (1): 1–5. doi:10.3852/mycologia.100.1.1. PMID 18488347.
- Sparrow, F.K. (1935). "Recent contributions to our knowledge of the aquatic phycomycetes". Biol. Rev. Camb. Philos. Soc. 10 (2): 152–186. doi:10.1111/j.1469-185X.1935.tb00480.x.
- Esser K (2014). The Mycota VII A: Systematics and Evolution (2nd ed.). Springer. p. 461. ISBN 978-3-642-55317-2.
- Powell; Letcher (2015). "A new genus and family for the misclassified chytrid, Rhizophlyctis harderi (in press)". Mycologia. 107 (2): 419–431. doi:10.3852/14-223. PMID 25572098. Retrieved 2016-08-23.
- Letcher, P.M.; Vélez, CG; Barrantes, M.E.; Powell, M.J.; Churchill, P.F.; Wakefield, W.S. (2008). "Ultrastructural and molecular analyses of Rhizophydiales (Chytridiomycota) isolates from North America and Argentina". Mycol. Res. 112 (Pt 7): 759–782. doi:10.1016/j.mycres.2008.01.025. PMID 18501579.
- Letcher, P.M.; Vélez, CG; Schultz, S; Powell, MJ (2012). "New taxa are delineated in Alphamycetaceae (Rhizophydiales, Chytridiomycota)". Nova Hedwigia. 94 (Pt 1–2): 9–29. doi:10.1127/0029-5035/2012/0094-0009.
- Hibbett, D.S.; et al. (March 2007). "A higher level phylogenetic classification of the Fungi". Mycological Research. 111 (5): 509–547. CiteSeerX 10.1.1.626.9582. doi:10.1016/j.mycres.2007.03.004. PMID 17572334.
- Longcore, J.E.; Letcher, P.M.; James, T.Y. (2011). "Homolaphlyctis polyrhiza gen. et sp. nov., a species in the Rhizophydiales (Chytridiomycetes) with multiple rhizoidal axes". Mycotaxon. 118: 433–440. doi:10.5248/118.433.
- Letcher, P.M.; Powell, M.J.; Viusent, M.C. (2008). "Rediscovery of an unusual chytridiaceous fungus new to the order Rhizophydiales". Mycologia. 100 (2): 325–334. doi:10.3852/mycologia.100.2.325. PMID 18592907.
- Lepelletier, F.; Karpov, S. A.; Alacid, E.; LePanse, S.; Bigeard, E.; Garces, E.; Jeanthon, C.; Guillou, L. (2014). "Dinomyces arenysensis gen. et sp. nov. (Rhizophydiales, Dinomycetaceae fam. nov.), a chytrid infecting marine dinoflagellates". Protist. 165 (2): 230–244. doi:10.1016/j.protis.2014.02.004. PMID 24709472.
- Letcher, P.M.; Powell, M.J.; Davis, W.J. (2015). "A new family and four new genera in Rhizophydiales (Chytridiomycota)". Mycologia. 107 (4): 808–830. doi:10.3852/14-280. PMID 25911694.
- Letcher, P.M.; Powell, M.J. (2005). "Kappamyces, a new genus in the Chytridiales (Chytridiomycota)". Nova Hedwigia. 80: 113–133. doi:10.1127/0029-5035/2005/0080-0115.
- Powell, M.J.; Letcher, P.M.; Longcore, J.E. (2011). "Operculomyces is a new genus in the order Rhizophydiales". Mycologia. 103 (4): 854–862. doi:10.3852/10-293. PMID 21262983.
- Powell, M.J.; Letcher, P.M.; Chambers, J.G.; Roychoudhury, S. (2015). "A new genus and family for the misclassified chytrid, Rhizophlyctis harderi". Mycologia. 107 (2): 419–431. doi:10.3852/14-223. PMID 25572098.
External links
| Wikimedia Commons has media related to Rhizophydiales. |