Rauhut–Currier reaction
The Rauhut–Currier reaction, also called the vinylogous Morita–Baylis–Hillman reaction,[1] is an organic reaction describing (in its original scope) the dimerization or isomerization of electron-deficient alkenes such as enones by action of an organophosphine of the type R3P.[2] In a more general description the RC reaction is any coupling of one active alkene / latent enolate to a second Michael acceptor, creating a new C–C bond between the alpha-position of one activated alkene and the beta-position of a second alkene under the influence of a nucleophilic catalyst.[3] The reaction mechanism is essentially that of the related and better known Baylis–Hillman reaction (DABCO not phosphine, carbonyl not enone) but the Rauhut–Currier reaction actually predates it by several years. In comparison to the MBH reaction, the RC reaction lacks substrate reactivity and reaction selectivity.
The original 1963 reaction described the dimerization of the ethyl acrylate to the ethyl diester of 2-methylene-glutaric acid with tributylphosphine in acetonitrile:
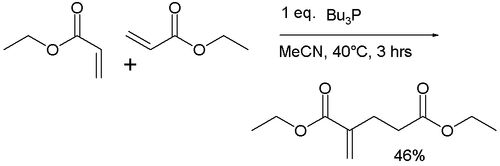
This reaction was also found to work for acrylonitrile.
RC cross-couplings are known but suffer from lack of selectivity. Amines such as DABCO can also act as catalyst. The reactivity is improved in intramolecular RC reactions, for example in the isomerization of di-enones to form cyclopentenes:[4]
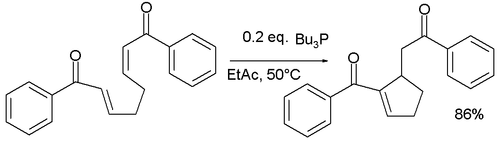
A similar reaction by asymmetric synthesis organocatalyzed by a protected cysteine and potassium tert-butoxide afforded a cyclohexene with 95% enantiomeric excess:[5]
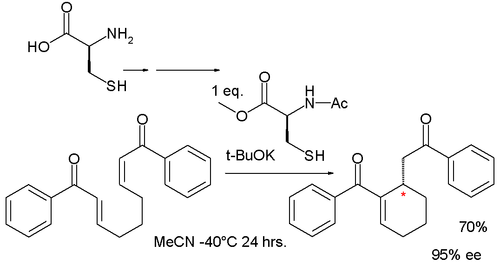
In this reaction the phosphine is replaced by the thiol group of cysteine but the reaction is the same.
References
- "The Vinylogous Intramolecular Morita−Baylis−Hillman Reaction: Synthesis of Functionalized Cyclopentenes and Cyclohexenes with Trialkylphosphines as Nucleophilic Catalysts" Scott A. Frank, Dustin J. Mergott, and William R. RoushJ. Am. Chem. Soc.; 2002; 124(11) pp. 2404–05; (Communication) doi:10.1021/ja017123j
- Preparation of dialkyl-2-methylene glutamates Rauhut, M. M.; Currier, H. U.S. Patent 3074999 1963 January 22, American Cyanamid Co., 1963. U.S. Patent 3,074,999
- The Rauhut–Currier reaction: a history and its synthetic application Carrie E. Aroyan, Alpay Dermenci and Scott J. Miller Tetrahedron Volume 65, Issue 21, 23 May 2009, pp. 4069–84 doi:10.1016/j.tet.2009.02.066
- Organocatalytic Michael Cycloisomerization of Bis(enones): The Intramolecular Rauhut-Currier Reaction Long-Cheng Wang, Ana Liza Luis, Kyriacos Agapiou, Hye-Young Jang, and Michael J. Krische J. Am. Chem. Soc.; 2002; 124(11) pp 2402 – 2403; (Communication) doi:10.1021/ja0121686
- Enantioselective Rauhut-Currier Reactions Promoted by Protected Cysteine Carrie E. Aroyan and Scott J. Miller J. Am. Chem. Soc.; 2007; 129(2) pp 256 – 257; (Communication) doi:10.1021/ja067139f