Mire
A mire, peatland or quagmire is a wetland type, dominated by living peat-forming plants. Mires arise because of incomplete decomposition of organic matter, usually litter from vegetation, due to water-logging and subsequent anoxia.[1] All types of mires share the common characteristic of being saturated with water at least seasonally with actively forming peat, while having its own set of vegetation and organisms.[2] Like coral reefs, mires are unusual landforms in that they derive mostly from biological rather than physical processes, and can take on characteristic shapes and surface patterning.
%E2%80%93Valle_Carbajal_01.jpg)
A quagmire is a floating (quaking) mire, bog or any peatland being in a stage of hydrosere or hydrarch (hydroseral) succession, resulting in pond-filling yields underfoot. Ombrotrophic types of quagmire may be called quaking bog (quivering bog). Minerotrophic types can be named with the term quagfen.[3]
There are four types of mire: bog, fen, marsh and swamp.[4] A bog is a mire that due to its location relative to the surrounding landscape obtains most of its water from rainfall (ombrotrophic), while a fen is located on a slope, flat, or depression and gets most of its water from soil- or groundwater (minerotrophic). Thus while a bog is always acidic and nutrient-poor, a fen may be slightly acidic, neutral, or alkaline, and either nutrient-poor or nutrient-rich.[5] Although marshes are wetlands within which vegetation is rooted in mineral soil, some marshes form shallow peat deposits: these should be considered mires. Swamps are characterised by their forest canopy and, like fens, are typically of higher pH and nutrient availability than bogs. Some bogs and fens can support limited shrub or tree growth on hummocks.
The formation of mires today is primarily controlled by climatic conditions, such as precipitation and temperature, although terrain relief is a major factor, as water-logging occurs more easily on flatter ground.[6] However, there is a growing anthropogenic influence in the accumulation of peat and peatlands around the world.[7]

Topographically, mires elevate the ground surface above the original topography. Mires can reach considerable heights above the underlying mineral soil or bedrock: peat depths of above 10 m have been commonly recorded in temperate regions (many temperate and most boreal mires were removed by ice sheets in the last Ice Age), and above 25 m in tropical regions.[7] When the absolute decay rate in the catotelm (the lower, water-saturated zone of a mire) matches the rate of input of new peat into the catotelm, the mire will stop growing in height.[8] A simplistic calculation, using typical values for a Sphagnum bog of 1 mm new peat added per year and 0.0001 proportion of the catotelm decaying per year, gives a maximum height of 10 m. More advanced analyses incorporate expectable nonlinear rates of catotelm decay.
For botanists and ecologists, the term peatland is a more general term for any terrain dominated by peat to a depth of at least 30 cm (12 in), even if it has been completely drained (i.e., a peatland can be dry, but a mire by definition must be actively forming peat).[1]
Global distribution



Mires, although perhaps at their greatest extent at high latitudes in the Northern Hemisphere, are found around the globe. Estimating the extent of mire land cover worldwide is difficult due to the varying accuracy and methodologies of land surveys from many countries.[6] However, mires occur wherever conditions are right for peat accumulation: largely where organic matter is constantly waterlogged. The distribution of mires therefore depends on topography, climate, parent material, biota and time.[9] The type of mire - bog, fen or swamp - depends also on each of these factors.
The largest accumulations of mires, constituting around 64% of global peatlands, are found in the temperate, boreal and subarctic zones of the Northern Hemisphere.[10] In polar regions, mires are usually shallow, because of the slow rate of accumulation of dead organic matter, and often contain permafrost. Very large swathes of Canada, northern Europe and northern Russia are covered by boreal mires. In temperate areas mires are typically more scattered due to historical drainage and peat extraction, but can cover large areas. One example is blanket bog where precipitation is very high (e.g. in maritime climates inland near the coasts of the north-east and south Pacific, and the north-west and north-east Atlantic). In the sub-tropics, mires are rare and restricted to the wettest areas.
In the tropics, mires can again be extensive, typically underlying tropical rainforest (for example, in Kalimantan), although tropical peat formation occurs in coastal mangroves, as well as in areas of high altitude.[7] Tropical mires largely form where high precipitation is combined with poor conditions for drainage.[6] Tropical mires account for around 11% of peatlands globally (more than half of which can be found in Southeast Asia), and are most commonly found at low altitudes, although they can also be found in mountainous regions, for example in South America, Africa and Papua New Guinea.[10] Recently, the world's largest tropical mire was found in the Central Congo Basin, covering 145,500 square kilometres and may store up to 30 petagrams of Carbon.[11]
Mires have declined globally due to drainage for agriculture and forestry, and for peat harvesting. For example, more than 50% of original European mire area, more than 300000 km2, has been lost.[12] Some of the largest losses have been in Russia, Finland, the Netherlands, the United Kingdom, Poland and Belarus.
Bio-chemical processes
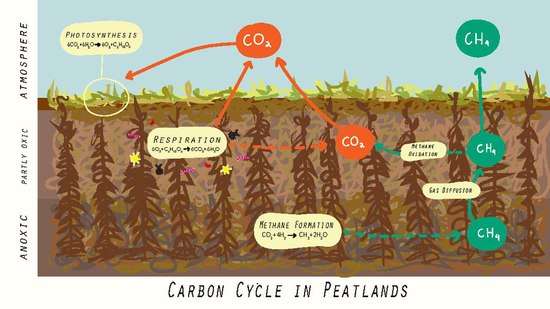
Mires have unusual chemistry, which influences inter alia their biota and the chemistry of the water outflow. Peat has very high cation-exchange capacity due to its high organic matter content: cations such as Ca2+ are preferentially adsorbed onto the peat in exchange for H+ ions. Water passing through peat declines in nutrients and in pH. Therefore mires are typically nutrient-poor and acidic unless the inflow of groundwater (bringing in supplementary cations) is high.[13]
Mires generally form whenever inputs of carbon exceed carbon outputs. This occurs due to the anoxic state of water-logged peat, and the process of photosynthesis by which peat grows.[14] Due to this, mires are collectively a major carbon store, containing between 500 and 700 billion tonnes of carbon, despite accounting for just 3% of Earth's land surfaces. Carbon stored within mires equates to over half the amount of carbon found in the atmosphere.[7] Mires interact with the atmosphere primarily through the exchange of carbon dioxide, methane and nitrous oxide.[1] The sequestration of carbon dioxide takes place at the surface via the process of photosynthesis, while losses of carbon dioxide occur through living peat tissue via respiration.[6] In their natural state, mires are a slight atmospheric carbon dioxide sink through the photosynthesis of peat vegetation, which outweighs their release of greenhouse gases. In addition, most mires are generally net emitters of methane and nitrous oxide.[15]
The water table position of a mire influences its carbon release to the atmosphere. When the water table rises, for example after a rainstorm, the peat and its microbes are submerged under water and access to oxygen is inhibited, reducing respiration and carbon dioxide release. Carbon dioxide release increases when the water table shrinks, such as during a drought, as this supplies the aerobic microbes with oxygen to decompose the peat.[16] Levels of methane also vary with the water table position and somewhat with temperature. A water table near the peat surface gives the opportunity for anaerobic microorganisms to flourish. Methanogens are responsible for producing methane via decomposition of the peat which consequently increases as the water table rises and oxygen levels are depleted. Increased temperatures in the soil also contributes to increased seasonal methane flux, though at a lower intensity. It is shown that the methane increased by as much as 300% seasonal from increased precipitation and temperature of the soil.[17]
Mires are important reservoirs of climatic information to the past because they are sensitive to changes in the environment and can reveal levels of isotopes, pollutants, macrofossils, metals from the atmosphere, and pollen.[18] For example, carbon-14 dating can reveal the age of the peat. The dredging and destruction of a mire will release the carbon dioxide that could reveal irreplaceable information about the past climatic conditions. It is widely known that a plethora of microorganisms inhabit mires due to the regular supply of water and abundance of peat forming vegetation. These microorganisms include but are not limited to methanogens, algae, bacteria, zoobenthos, of which Sphagnum species are most abundant.[19] The peat in mires contain a substantial amount of organic matter, where humic acid dominates. Humic materials are able to store very large amounts of water, making them an essential component in the peat environment, contributing to an increased amount of carbon storage due to the resulting anaerobic condition. If the peatland is dried from long-term cultivation and agricultural use, it will lower the water table and the increased aeration will subsequently release carbon content.[20] Upon extreme drying, the ecosystem can undergo a state shift, turning the mire into a barren land with lower biodiversity and richness. The formation of humic acid occurs during the biogeochemical degradation of vegetation debris, animal residue, and degraded segments.[21] The loads of organic matter in the form of humic acid is a source of precursors of coal. Prematurely exposing the organic matter to the atmosphere promotes the conversion of organics to carbon dioxide to be released in the atmosphere.
Anthropogenic uses
Mires are used by humans for a range of purposes, the most dominant being agriculture and forestry, which accounts for around a quarter of global peatland area.[7] This involves cutting drainage ditches to lower the water table with the intended purpose of enhancing the productivity of forest cover or for use as pasture or cropland.[1] Agricultural uses for mires include the use of natural vegetation for hay crop or grazing, or the cultivation of crops on a modified surface.[6] In addition, the commercial harvest of peat from mires for energy production is widely practiced in Northern European countries, such as Russia, Sweden, Finland and the Baltic states.[7]
The clearing of tropical mires for anthropogenic uses is an increasingly pressing issue in Southeast Asia, where opportunities for the production of palm oil and timber for export are leading primarily developing nations to exploit mires for economic purposes.[10] Tropical peatlands comprise 0.25% of Earth’s terrestrial land surface but store 3% of all soil and forest carbon stocks and are mostly located in low-income countries. The exploitation of these ecosystems, such as the draining and harvesting of tropical peat forests, continues to result in the emission of large amounts of carbon dioxide into the atmosphere. In addition, fires occurring on peatland dried by the draining of peat bogs releases even more carbon dioxide. The economic value of a tropical peatland used to be derived from raw materials, such as wood, bark, resin, and latex; the extraction of which did not contribute to large carbon emissions. Today, many of these ecosystems are drained for conversion to palm oil plantations, releasing the stored carbon dioxide and preventing the system from sequestering carbon again. The planned Carbopeat Project will attempt to assign economic value to the carbon sequestration performed by peat bogs to stop the exploitation of these ecosystems.[22]
Moreover, records of past human behaviour and environments can be contained within mires. These may take the form of human artefacts, or paleoecological and geochemical records.[7]
Tropical mires
The global distribution of tropical mires is mostly concentrated to Southeast Asia where agricultural use of peatlands has been developed in recent decades. Large areas of tropical peatlands have been cleared and drained for food and cash crops such as palm oil plantation. Large scale drainage of these plantations often results in subsidence, flooding, fire and deterioration in soil quality. Small scale encroachment on the other hand, is linked to poverty and is so wide spread that it as well has a negative impact on these peatlands. The biotic and abiotic factors controlling the Southeast Asian peatlands are completely interdependent.[6] Its soil, hydrology and morphology are created by the present vegetation through the accumulation of its own organic matter where it builds a favorable environment for this specific vegetation. This system is therefore vulnerable to changes in hydrology or vegetation cover.[23] Furthermore, these peatlands are mostly located in developing regions with impoverished and rapidly growing populations. The lands have there for become target for commercial logging, paper pulp production and conversion to plantations through clear-cutting, drainage and burning.[6] Drainage of tropical peatlands alters the hydrology and increases their susceptibility to fire and soil erosion, as a consequence of changes in physical and chemical compositions.[24] The change in soil strongly effects the sensitive vegetation and forest die-off is common. The short-term effect is a decrease in biodiversity but the long-term effect, since these encroachments are hard to reverse, is a loss of habitat. Poor knowledge about peatlands sensitive hydrology and lack of nutrients often lead to failing plantations where pressure increases on remaining peatlands.[6]
Sustainable forestry in these peatlands is possible by cutting large trees and letting smaller individuals flourish but instead clear-cutting and burning to enable monocultural plantation of non-indigenous species is the predominant strategy.[6]
Northern peatlands were mostly built up during Holocene after the retreat of Pleistocene glaciers but in contrast the tropical ones are often much older. Nakaikemi Wetland in southwest Honshu, Japan is more than 50,000 years old and has a depth of 45 m.[6] The Philippi Peatland in Greece has probably one of the deepest peat layers with a depth of 190 m.[25] Tropical peatlands are suggested to contain about 100 Gt carbon[26][24] and is corresponding to more than 50% of the carbon present as CO2 in the atmosphere.[6] Accumulation rates of carbon during the last millennium were close to 40 g C/m2/yr.[27]
Greenhouse gases and fires
The tropical peatlands in Southeast Asia only cover 0,2% of earths land area but CO2 emissions are estimated to 2 Gt per year which is equal to 7% of the global fossil fuel emissions.[23] These emissions get bigger with drainage and burning of peatlands and a severe fire can release up to 4000 t of CO2/ha. Burning events in tropical peatlands are becoming more frequent due to large scale drainage and land clearance and in the past 10 years, more than 2 million ha was burnt in Southeast Asia alone. These fires last typically for 1–3 months and are releasing large amounts of CO2. Indonesia is one of the countries suffering from peatland fires, especially during years with ENSO-related drought, an increasing problem since 1982 as a result of developing land use and agriculture.[24] During the El Niño-event in 1997-1998 more than 24,400 km2[6] of peatland was lost to fires in Indonesia alone from which 10,000 km2 was burnt in Kalimantan and Sumatra. The output of CO2 was estimated to 0.81–2.57 Gt, equal to 13–40% of global output from fossil fuel burning. Indonesia is now considered the 3rd biggest contributor to global CO2 emissions, caused primarily by these fires.[28] With a warming climate these burnings are expected to increase in intensity and number. This is a result of a dry climate together with an extensive rice farming project, called The Mega Rice Project, started in the 1990s where 1 Mha of peatlands was converted to rice paddies. Forest and land was cleared by burning and 4000 km of channels drained the area.[29] Drought and acidification of the lands led to bad harvest and the project was abandoned in 1999.[30] Similar projects in China have led to immense loss of tropical marshes and fens due to rice production.[31] Drainage, which also increases the risk of burning, can cause additional emissions of CO2 by 30–100 t/ha/year if the water table is lowered with only 1 m.[32] The draining of peatlands is probably the most important and long lasting threat to peatlands all over the world but especially in the tropics.[24] Peatlands do release the greenhouse gas methane that has strong global warming potential, but subtropical wetlands have shown high CO2 binding per mol of released methane, which is a function that counteracts global warming.[33]
Biology and peat characteristics
The vegetation of tropical peatlands varies with climate and location. Three different characterizations are mangrove woodlands present in the littoral zones and deltas of salty water, followed inland by swamp forests. These forests occur on the margin of peatlands with a palm rich flora with trees 70 m tall and 8 m in girth accompanied by ferns and epiphytes. The third one, Padang, from the Malaysian and Indonesian word for forest, consists of shrubs and tall but thin trees and appear in the center of large peatlands.[6] The diversity of woody species, like trees and shrubs, are far greater in the tropical peatlands than in peatlands of other types. The peat in the tropics is therefore dominated by woody material from trunks of trees and shrubs and contain little to no sphagnum moss that dominates in boreal peatlands.[6] It’s only partly decomposed and the surface consists of a thick layer of leaf litter.[6] Forestry in peatlands leads to drainage and rapid carbon losses since it decreases inputs of organic matter and accelerate the decomposition.[34] In contrast to temperate wetlands the tropical peatlands are home to several species of fish. Many new, often endemic, species has been discovered lately[35] but many of them are considered threatened.[24]
Impacts on global climate
Wetlands provide an environment where organic carbon is stored in living plants, dead plants and peat, as well as converted to carbon dioxide and methane. Three main factors giving wetlands the ability to sequester and store carbon are the high biological productivity, high water table and low decomposition rates. Suitable meteorological and hydrological conditions are necessary to provide an abundant water source for the wetland. Fully water-saturated wetland soils allow anaerobic conditions to manifest, storing carbon but releasing methane.[36]
Wetlands make up about 5-8% of Earth’s terrestrial land surface but contain about 20-30% of the planet’s 2500 Gt soil carbon stores.[37] Mires, (e.g., bogs, fens and marshes) are the wetland types that contain the highest amounts of soil organic carbon, and can thus be considered peatlands (a peat layer >30 cm).[38] Wetlands can become sources of carbon, rather than sinks, as the decomposition occurring within the ecosystem emits methane.[36] Natural peatlands do not always have a measurable cooling effect on the climate in a short time span as the cooling effects of sequestering carbon are offset by the emission of methane, which is a strong greenhouse gas. However, given the short "lifetime" of methane (12 years), it is often said that methane emissions are unimportant within 300 years compared to carbon sequestration in wetlands. Within that time frame or less, most wetlands become both net carbon and radiative sinks. Hence, peatlands do result in cooling of the Earth's climate over a longer time period as methane is oxidised quickly and removed from the atmosphere whereas atmospheric carbon dioxide is continuously absorbed.[39] Throughout the Holocene (the past 12,000 years), peatlands have been persistent terrestrial carbon sinks and have had a net cooling effect, sequestering 5.6 to 38 grams of carbon per square metre per year. Today, it has been estimated that northern peatlands, on average, sequester 20-30 grams of carbon per square meter per year.[1][40]
Peatlands insulate the permafrost in subarctic regions, thus delaying thawing during summer, as well as inducing the formation of permafrost.[39] As the global climate continues to warm, wetlands could become major carbon sources as higher temperatures cause higher carbon dioxide emissions.[41]
Compared with untilled cropland, wetlands can sequester around two times the carbon, and planted wetlands may be able to store 2-15 times more carbon than what they release. Carbon sequestration can occur in constructed wetlands, as well as natural ones. Estimates of greenhouse gas fluxes from wetlands indicate that natural wetlands have lower fluxes, but man-made wetlands have a greater carbon sequestration capacity. The carbon sequestration abilities of wetlands can be improved through restoration and protection strategies, but it takes several decades for these restored ecosystems to become comparable in carbon storage to peatlands and other forms of natural wetlands.[36]
Effects of drainage for agriculture and forestry
Due to their significance in the global soil-atmosphere exchange of carbon, the movement of carbon between mires and the atmosphere is an important current issue in ecology and biogeochemical studies.[6] The drainage of peatlands for agriculture and forestry has resulted in the emission of extensive greenhouse gasses into the atmosphere, most notably carbon dioxide and methane. By allowing oxygen to enter the peat column within a mire, drainage disrupts the balance between peat accumulation and decomposition, and the subsequent oxidative degradation results in the release of carbon into the atmosphere.[42] As such, the drainage of mires for agriculture transforms them from net carbon sinks, to net carbon emitters.[1] However, the emission of methane from mires has been observed to decrease following drainage.[15]
When undertaken in such a way that preserves the hydrological state of a mire, the anthropogenic use of mires' resources can avoid significant greenhouse gas emissions. However, continued drainage will result in increased release of carbon, contributing to global warming. As of 2016, it was estimated that drained peatlands account for around 10% of all greenhouse gas emissions from agriculture and forestry.[7]
Fires
Mire drainage or drying due to climactic factors may also increase the risk of fires, presenting further risk of carbon and methane release into the atmosphere.[7] Due to their naturally high moisture content, pristine mires have a generally low risk of fire ignition. The drying of this waterlogged state means that the carbon-dense vegetation becomes vulnerable to fire. In addition, the oxygen poor nature of the vegetation causes peat fires to smoulder beneath the surface, causing incomplete combustion of the organic matter and resulting in extreme emissions events.[7]
In recent years, the occurrence of wildfires in peatlands has increased significantly worldwide, but particularly in tropical regions. This can be attributed to a combination of drier weather and changes in land use which involve the drainage of water from the landscape.[1] This resulting loss of biomass through combustion has led to significant emissions of greenhouse gasses both in tropical and boreal/temperate peatlands.[43] Fire events are predicted become more frequent with the warming and drying of the global climate.[6]
Palm oil plantations
Oil palm is increasingly becoming one of the world’s largest crops, rapidly expanding in the past years. In comparison to alternatives, oil palm is considered to be among the most efficient sources of vegetable oil and biofuel, requiring only 0.26 hectares of land to produce 1 ton of oil.[44] Thus, palm oil has become a popular cash crop in many low-income countries, providing economic opportunities for communities. With palm oil as a leading export in countries such as Indonesia and Malaysia, many smallholders have found economic success in palm oil plantations. However, the land sequestered for plantations are typically substantial carbon stores promoting biodiverse ecosystems.[45]
Oil palm plantations have replaced much of the forested peatlands in Southeast Asia. Historically, these regions have been seen as a dead space, but estimates now state that 12.9 Mha, or about 47% of peatlands in Southeast Asia, were deforested by 2006.[46] In their natural state, peatlands are waterlogged, with high water tables, making for an inefficient soil.[44] To create viable soil for plantation, the mires in tropical regions of Indonesia and Malaysia are drained and cleared.
The peatland forests being harvested for palm oil production serve as above and below ground carbon stores, containing at least 42,000 Million metric tonnes (Mt) of soil carbon.[46] This exploitation of land raises many environmental concerns, namely greenhouse gas emissions, risk of fires, and a decrease in biodiversity. The greenhouse gas emissions for palm oil planted on peatlands is estimated to be between the equivalent of 12.4 (best case) to 76.6 t CO2/ha (worst case).[44]
In their natural state, peatlands are resistant to fire. Drainage of peatlands for palm oil plantations creates a dry layer of peat that is especially vulnerable to fires. As peat is carbon dense, fires occurring in compromised peatlands release extreme amounts of both carbon dioxide and toxic smoke into the air. Thus, these fires not only add to emissions of greenhouse gases, but also cause thousands of deaths every year.
The decrease in biodiversity, due to deforestation and drainage, creates a vulnerable ecosystem. Homogenous ecosystems are at an increased risk to extreme climate conditions, and are less likely to recover from fires.
Management and rehabilitation
Rehabilitation projects undertaken in North America and Europe usually focus around the rewetting of peatlands and revegetation with native species. This acts to mitigate carbon release in the short term, before the new vegetation growth provides a new source of organic litter to fuel the peat formation process in the long term.[7]
The United Nations Convention of Biological Diversity targets highlights peatlands as key ecosystems to be conserved and protected. The conventions requires governments at all levels to present action plans for the conservation and management of wetland environments. Wetlands are also protected under the 1971 Ramsar Convention.[7]
References
- Frolking, Steve; Talbot, Julie; Jones, Miriam C.; Treat, Claire C.; Kauffman, J. Boone; Tuittila, Eeva-Stiina; Roulet, Nigel (December 2011). "Peatlands in the Earth's 21st century climate system". Environmental Reviews. 19 (NA): 371–396. doi:10.1139/a11-014. ISSN 1181-8700.
- "Wetlands Types and Classifications". Retrieved 20 May 2019.
- https://pub.epsilon.slu.se/3014/1/SFS205.pdf
- National Wetlands Working Group (1997). The Canadian wetland classification system (2nd ed.). University of Waterloo, Canada.
- Geist, Helmut (2006). Our Earth's Changing Land: An Encyclopedia of Land-Use and Land-Cover Change. 2. Greenwood. p. 463. ISBN 9780313327841.
- Rydin, Håkan. (2013). The biology of peatlands. Jeglum, J. K., Bennett, Keith D. (2nd ed.). Oxford: Oxford University Press. ISBN 978-0199602995. OCLC 840132559.
- Page, S.E.; Baird, A.J. (November 2016). "Peatlands and Global Change: Response and Resilience". Annual Review of Environment and Resources. 41 (1): 35–57. doi:10.1146/annurev-environ-110615-085520. ISSN 1543-5938.
- Joosten H.; Tanneberger F.; Moen, A., eds. (2017). Mires and Peatlands of Europe. Schweizerbart Science Publishers. Stuttgart.
- Gorham, Eville (1857). "The Development of Peat Lands". The Quarterly Review of Biology. 32 (2): 145–166. doi:10.1086/401755.
- PAGE, SUSAN E.; RIELEY, JOHN O.; BANKS, CHRISTOPHER J. (2011-01-04). "Global and regional importance of the tropical peatland carbon pool" (PDF). Global Change Biology. 17 (2): 798–818. Bibcode:2011GCBio..17..798P. doi:10.1111/j.1365-2486.2010.02279.x. ISSN 1354-1013.
- Dargie, Greta C.; Lewis, Simon L.; Lawson, Ian T.; Mitchard, Edward T. A.; Page, Susan E.; Bocko, Yannick E.; Ifo, Suspense A. (2017-01-11). "Age, extent and carbon storage of the central Congo Basin peatland complex" (PDF). Nature. 542 (7639): 86–90. Bibcode:2017Natur.542...86D. doi:10.1038/nature21048. ISSN 0028-0836. PMID 28077869.
- Joosten, H.; Clarke, D. (2002). Wise use of mires and peatlands. International Mire Conservation Group and International Peat Society.
- Rydin, Håkan; Jeglum, John (2006). The Biology of Peatlands (1st ed.). Oxford University Press.
- Belyea, Lisa R.; Malmer, Nils (July 2004). "Carbon sequestration in peatland: patterns and mechanisms of response to climate change". Global Change Biology. 10 (7): 1043–1052. Bibcode:2004GCBio..10.1043B. doi:10.1111/j.1529-8817.2003.00783.x.
- "News and Views". Scandinavian Journal of Forest Research. 16 (4): 289–294. 2001-07-01. doi:10.1080/02827580120112. ISSN 0000-0000.
- Brown, Alastair (2011-12-20). "Carbon storage: When peat dries". Nature Climate Change. 2 (1): 22. doi:10.1038/nclimate1360.
- Turetsky, M. R.; Treat, C. C.; Waldrop, M. P.; Waddington, J. M.; Harden, J. W.; McGuire, A. D. (2008-09-01). "Short-term response of methane fluxes and methanogen activity to water table and soil warming manipulations in an Alaskan peatland". Journal of Geophysical Research. 113 (G3): G00A10. Bibcode:2008JGRG..113.0A10T. doi:10.1029/2007jg000496. ISSN 2156-2202.
- Tobolski, K (2000). Przewodnik do oznaczania torfów i osadów jeziornych. PWN.
- Kuske, E; Silamikele, Inese; Kalnina, Laimdota; Klavins, Maris (2010-01-01). "Peat formation conditions and peat properties: A study of two ombrotrophic bogs in Latvia". Mires and Peat.
- Environment, Szajdak, L., Polish Academy of Sciences, Poznan (Poland). Inst. for Agricultural and Forest; Improvement, Szatylowicz, J., Warsaw Univ. of Life Sciences (Poland). Dept. of Environmental (2010). Impact of drainage on hydrophobicity of fen peat-moorsh soils. AGRIS: International Information System for the Agricultural Science and Technology. University of Latvia Press. ISBN 9789984451633.
- Chemistry, Gierlach-Hladon, T., Karol Marcinkowski Univ. of Medical Sciences, Poznan (Poland). Dept. of Inorganic and Analytical; Environment, Szajdak, L., Polish Academy of Sciences, Poznan (Poland). Inst. for Agricultural and Forest (2010). Physico-chemical properties of humic acids isolated from an Eriophorum-Sphagnum raised bog. AGRIS: International Information System for the Agricultural Science and Technology. University of Latvia Press. ISBN 9789984451633.
- "Carbon sequestration in peat bogs as a source of income". WUR. Retrieved 2018-04-09.
- Hooijer, A., Silvius, M., Wösten, H. and Page, S. 2006. PEAT-CO2 , Assessment of CO2 emissions from drained peatlands in SE Asia. Delft Hydraulics report Q3943.
- United Nations Environment Programme. Global Environment Facility. Asia Pacific Network for Global Change Research. Global Environment Centre (Malaysia), publisher. Wetlands International, publisher. Assessment on peatlands, biodiversity, and climate change. ISBN 9789834375102. OCLC 933580381.CS1 maint: multiple names: authors list (link)
- Christanis, Kimon (2016), Finlayson, C. Max; Milton, G. Randy; Prentice, R. Crawford; Davidson, Nick C. (eds.), "The Wetland Book", The Wetland Book: II: Distribution, Description and Conservation, Springer Netherlands, pp. 1–6, doi:10.1007/978-94-007-6173-5_147-1, ISBN 9789400761735
|chapter=ignored (help) - Peatlands and climate change. Strack, Maria., International Peat Society. Jyväskylä, Finland: IPS, International Peat Society. 2008. ISBN 9789529940110. OCLC 404026180.CS1 maint: others (link)
- Yu, Zicheng; Loisel, Julie; Brosseau, Daniel P.; Beilman, David W.; Hunt, Stephanie J. (July 2010). "Global peatland dynamics since the Last Glacial Maximum". Geophysical Research Letters. 37 (13): n/a. Bibcode:2010GeoRL..3713402Y. doi:10.1029/2010gl043584. ISSN 0094-8276.
- Silvius, M., Kaat, A.H., Van de Bund and Hooijer, A. 2006. Peatland degradation fuels climate change. An unrecognised and alarming source of greenhouse gases. Wetlands International, Wageningen, The Netherlands.
- Boehm, H.-D. V., Siegert, F., Rieley, J. O. et al (2001). Fire impacts and carbon release on tropical peatlands in central Kalimantan, Indonesia. 22nd Asian Conference on Remote Sensing, 5–9 November 2001, Singapore. Centre for Remote Imaging, Sensing and Processing (CRISP), University of Singapore.
- Page, Susan; Hoscilo, Agata; Langner, Andreas; Tansey, Kevin; Siegert, Florian; Limin, Suwido; Rieley, Jack (2009), "Tropical peatland fires in Southeast Asia", Tropical Fire Ecology, Springer Berlin Heidelberg, pp. 263–287, doi:10.1007/978-3-540-77381-8_9, ISBN 9783540773801
- "'94 International Conference on Wetland Environment and Peatland Utilization". Chinese Geographical Science. 4 (1): 95. March 1994. doi:10.1007/bf02664953. ISSN 1002-0063.
- Wösten, J. H. M.; Van Den Berg, J.; Van Eijk, P.; Gevers, G. J. M.; Giesen, W. B. J. T.; Hooijer, A.; Idris, Aswandi; Leenman, P. H.; Rais, Dipa Satriadi (March 2006). "Interrelationships between Hydrology and Ecology in Fire Degraded Tropical Peat Swamp Forests". International Journal of Water Resources Development. 22 (1): 157–174. doi:10.1080/07900620500405973. ISSN 0790-0627.
- WHITING, GARY J.; CHANTON, JEFFREY P. (November 2001). "Greenhouse carbon balance of wetlands: methane emission versus carbon sequestration". Tellus B. 53 (5): 521–528. Bibcode:2001TellB..53..521W. doi:10.1034/j.1600-0889.2001.530501.x. ISSN 0280-6509.
- Biodiversity and sustainability of tropical peatlands : proceedings of the International Symposium on Biodiversity, Environmental Importance and Sustainability of Tropical Peat and Peatlands, held in Palangka Raya, Central Kalimantan, Indonesia, 4-8 September 1995. Rieley, Jack, 1941-, Page, Susan, 1957-. Cardigan, UK: Samara Pub. 1997. ISBN 1873692102. OCLC 37815652.CS1 maint: others (link)
- Ng, Peter K. L.; Tay, J. B.; Lim, Kelvin K. P. (1994), "Diversity and conservation of blackwater fishes in Peninsular Malaysia, particularly in the North Selangor peat swamp forest", Ecology and Conservation of Southeast Asian Marine and Freshwater Environments including Wetlands, Springer Netherlands, pp. 203–218, doi:10.1007/978-94-011-0958-1_20, ISBN 9789401044141
- Kayranli, Birol; Scholz, Miklas; Mustafa, Atif; Hedmark, Åsa (2010-02-01). "Carbon Storage and Fluxes within Freshwater Wetlands: a Critical Review". Wetlands. 30 (1): 111–124. doi:10.1007/s13157-009-0003-4. ISSN 0277-5212.
- Mitsch, William J.; Bernal, Blanca; Nahlik, Amanda M.; Mander, Ülo; Zhang, Li; Anderson, Christopher J.; Jørgensen, Sven E.; Brix, Hans (2013-04-01). "Wetlands, carbon, and climate change". Landscape Ecology. 28 (4): 583–597. doi:10.1007/s10980-012-9758-8. ISSN 0921-2973.
- Köchy, M.; Hiederer, R.; Freibauer, A. (2015-04-16). "Global distribution of soil organic carbon – Part 1: Masses and frequency distributions of SOC stocks for the tropics, permafrost regions, wetlands, and the world". SOIL. 1 (1): 351–365. Bibcode:2015SOIL....1..351K. doi:10.5194/soil-1-351-2015. ISSN 2199-3971.
- "Peatlands, climate change mitigation and biodiversity conservation | Ramsar". www.ramsar.org. Retrieved 2018-04-09.
- Yu, Zicheng; Beilman, D. W.; Frolking, S.; MacDonald, G. M.; Roulet, N. T.; Camill, P.; Charman, D. J. (2011). "Peatlands and Their Role in the Global Carbon Cycle". Eos, Transactions American Geophysical Union. 92 (12): 97–98. Bibcode:2011EOSTr..92...97Y. doi:10.1029/2011EO120001. ISSN 2324-9250.
- Turetsky, Merritt R.; Abbott, Benjamin W.; Jones, Miriam C.; Walter Anthony, Katey; Olefeldt, David; Schuur, Edward A. G.; Koven, Charles; McGuire, A. David; Grosse, Guido (2019-04-30). "Permafrost collapse is accelerating carbon release". Nature. 569 (7754): 32–34. Bibcode:2019Natur.569...32T. doi:10.1038/d41586-019-01313-4. ISSN 0028-0836. PMID 31040419.
- Minkkinen, Kari; Laine, Jukka (1998). "Long-term effect of forest drainage on the peat carbon stores of pine mires in Finland". Canadian Journal of Forest Research. 28 (9): 1267–1275. doi:10.1139/x98-104.
- Granath, Gustaf; Moore, Paul A.; Lukenbach, Maxwell C.; Waddington, James M. (2016-06-27). "Mitigating wildfire carbon loss in managed northern peatlands through restoration". Scientific Reports. 6 (1): 28498. Bibcode:2016NatSR...628498G. doi:10.1038/srep28498. ISSN 2045-2322. PMC 4921962. PMID 27346604.
- Hashim, Zulkifli; Subramaniam, Vijaya; Harun, Mohd Haniff; Kamarudin, Norman (June 2018). "Carbon footprint of oil palm planted on peat in Malaysia". The International Journal of Life Cycle Assessment. 23 (6): 1201–1217. doi:10.1007/s11367-017-1367-y. ISSN 0948-3349.
- LAURANCE, WILLIAM F.; KOH, LIAN P.; BUTLER, RHETT; SODHI, NAVJOT S.; BRADSHAW, COREY J. A.; NEIDEL, J. DAVID; CONSUNJI, HAZEL; MATEO VEGA, JAVIER (April 2010). "Improving the Performance of the Roundtable on Sustainable Palm Oil for Nature Conservation". Conservation Biology. 24 (2): 377–381. doi:10.1111/j.1523-1739.2010.01448.x. ISSN 0888-8892. PMID 20184655.
- Hooijer, A.; Page, S.; Canadell, J. G.; Silvius, M.; Kwadijk, J.; Wösten, H.; Jauhiainen, J. (2010-05-12). "Current and future CO2 emissions from drained peatlands in Southeast Asia". Biogeosciences. 7 (5): 1505–1514. doi:10.5194/bg-7-1505-2010. ISSN 1726-4189.
External links
| Look up quagmire, mire, or peatland in Wiktionary, the free dictionary. |
- . Encyclopædia Britannica. 22 (11th ed.). 1911. p. 703.