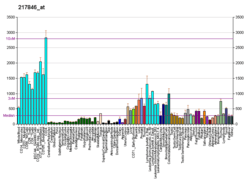
QARS
Glutaminyl-tRNA synthetase is an enzyme that in humans is encoded by the QARS gene.[3][4][5]
Function
Aminoacyl-tRNA synthetases catalyze the aminoacylation of tRNA by their cognate amino acid. Because of their central role in linking amino acids with nucleotide triplets contained in tRNAs, aminoacyl-tRNA synthetases are thought to be among the first proteins that appeared in evolution. In metazoans, 9 aminoacyl-tRNA synthetases specific for glutamine (gln), glutamic acid (glu), and 7 other amino acids are associated within a multienzyme complex. Although present in eukaryotes, glutaminyl-tRNA synthetase (QARS) is absent from many prokaryotes, mitochondria, and chloroplasts, in which Gln-tRNA(Gln) is formed by transamidation of the misacylated Glu-tRNA(Gln). Glutaminyl-tRNA synthetase belongs to the class-I aminoacyl-tRNA synthetase family.[5] Almost all eukaryotic GlnRS enzymes possess a YqeY domain at the N-terminus, which affects affinity for the tRNA; in some bacterial species, such as Deinococcus radiodurans, YqeY is present as a C-terminal domain with similar function.[6]
Interactions
QARS has been shown to interact with RARS.[7]
References
- GRCh38: Ensembl release 89: ENSG00000172053 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Lamour V, Quevillon S, Diriong S, N'Guyen VC, Lipinski M, Mirande M (Aug 1994). "Evolution of the Glx-tRNA synthetase family: the glutaminyl enzyme as a case of horizontal gene transfer". Proceedings of the National Academy of Sciences of the United States of America. 91 (18): 8670–4. Bibcode:1994PNAS...91.8670L. doi:10.1073/pnas.91.18.8670. PMC 44668. PMID 8078941.
- Durkin ME, Jäger AC, Khurana TS, Nielsen FC, Albrechtsen R, Wewer UM (July 1999). "Characterization of the human laminin beta2 chain locus (LAMB2): linkage to a gene containing a nonprocessed, transcribed LAMB2-like pseudogene (LAMB2L) and to the gene encoding glutaminyl tRNA synthetase (QARS)". Cytogenetics and Cell Genetics. 84 (3–4): 173–8. doi:10.1159/000015249. PMID 10393422.
- "Entrez Gene: QARS glutaminyl-tRNA synthetase".
- Hadd A, Perona JJ (Oct 2014). "Coevolution of specificity determinants in eukaryotic glutamyl- and glutaminyl-tRNA synthetases". Journal of Molecular Biology. 426 (21): 3619–33. doi:10.1016/j.jmb.2014.08.006. PMID 25149203.
- Kim T, Park SG, Kim JE, Seol W, Ko YG, Kim S (Jul 2000). "Catalytic peptide of human glutaminyl-tRNA synthetase is essential for its assembly to the aminoacyl-tRNA synthetase complex". The Journal of Biological Chemistry. 275 (28): 21768–72. doi:10.1074/jbc.M002404200. PMID 10801842.
Further reading
- Norcum MT (Aug 1991). "Structural analysis of the high molecular mass aminoacyl-tRNA synthetase complex. Effects of neutral salts and detergents". The Journal of Biological Chemistry. 266 (23): 15398–405. PMID 1651330.
- Maruyama K, Sugano S (Jan 1994). "Oligo-capping: a simple method to replace the cap structure of eukaryotic mRNAs with oligoribonucleotides". Gene. 138 (1–2): 171–4. doi:10.1016/0378-1119(94)90802-8. PMID 8125298.
- Suzuki Y, Yoshitomo-Nakagawa K, Maruyama K, Suyama A, Sugano S (Oct 1997). "Construction and characterization of a full length-enriched and a 5'-end-enriched cDNA library". Gene. 200 (1–2): 149–56. doi:10.1016/S0378-1119(97)00411-3. PMID 9373149.
- Quevillon S, Robinson JC, Berthonneau E, Siatecka M, Mirande M (Jan 1999). "Macromolecular assemblage of aminoacyl-tRNA synthetases: identification of protein-protein interactions and characterization of a core protein". Journal of Molecular Biology. 285 (1): 183–95. doi:10.1006/jmbi.1998.2316. PMID 9878398.
- Ko YG, Kang YS, Kim EK, Park SG, Kim S (May 2000). "Nucleolar localization of human methionyl-tRNA synthetase and its role in ribosomal RNA synthesis". The Journal of Cell Biology. 149 (3): 567–74. doi:10.1083/jcb.149.3.567. PMC 2174846. PMID 10791971.
- Kim T, Park SG, Kim JE, Seol W, Ko YG, Kim S (Jul 2000). "Catalytic peptide of human glutaminyl-tRNA synthetase is essential for its assembly to the aminoacyl-tRNA synthetase complex". The Journal of Biological Chemistry. 275 (28): 21768–72. doi:10.1074/jbc.M002404200. PMID 10801842.
- Kang J, Kim T, Ko YG, Rho SB, Park SG, Kim MJ, Kwon HJ, Kim S (Oct 2000). "Heat shock protein 90 mediates protein-protein interactions between human aminoacyl-tRNA synthetases". The Journal of Biological Chemistry. 275 (41): 31682–8. doi:10.1074/jbc.M909965199. PMID 10913161.
- Ko YG, Kim EY, Kim T, Park H, Park HS, Choi EJ, Kim S (Feb 2001). "Glutamine-dependent antiapoptotic interaction of human glutaminyl-tRNA synthetase with apoptosis signal-regulating kinase 1". The Journal of Biological Chemistry. 276 (8): 6030–6. doi:10.1074/jbc.M006189200. PMID 11096076.
- Lehner B, Semple JI, Brown SE, Counsell D, Campbell RD, Sanderson CM (Jan 2004). "Analysis of a high-throughput yeast two-hybrid system and its use to predict the function of intracellular proteins encoded within the human MHC class III region". Genomics. 83 (1): 153–67. doi:10.1016/S0888-7543(03)00235-0. PMID 14667819.
- Colland F, Jacq X, Trouplin V, Mougin C, Groizeleau C, Hamburger A, Meil A, Wojcik J, Legrain P, Gauthier JM (Jul 2004). "Functional proteomics mapping of a human signaling pathway". Genome Research. 14 (7): 1324–32. doi:10.1101/gr.2334104. PMC 442148. PMID 15231748.
- Rush J, Moritz A, Lee KA, Guo A, Goss VL, Spek EJ, Zhang H, Zha XM, Polakiewicz RD, Comb MJ (Jan 2005). "Immunoaffinity profiling of tyrosine phosphorylation in cancer cells". Nature Biotechnology. 23 (1): 94–101. doi:10.1038/nbt1046. PMID 15592455.
- Rual JF, Venkatesan K, Hao T, Hirozane-Kishikawa T, Dricot A, Li N, Berriz GF, Gibbons FD, Dreze M, Ayivi-Guedehoussou N, Klitgord N, Simon C, Boxem M, Milstein S, Rosenberg J, Goldberg DS, Zhang LV, Wong SL, Franklin G, Li S, Albala JS, Lim J, Fraughton C, Llamosas E, Cevik S, Bex C, Lamesch P, Sikorski RS, Vandenhaute J, Zoghbi HY, Smolyar A, Bosak S, Sequerra R, Doucette-Stamm L, Cusick ME, Hill DE, Roth FP, Vidal M (Oct 2005). "Towards a proteome-scale map of the human protein-protein interaction network". Nature. 437 (7062): 1173–8. Bibcode:2005Natur.437.1173R. doi:10.1038/nature04209. PMID 16189514.