Pseudoisoeugenol
Pseudoisoeugenol is a naturally occurring phenylpropene and an isomer of eugenol.[1]
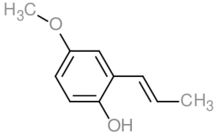 | |
| Names | |
|---|---|
| Preferred IUPAC name
4-Methoxy-2-[(E)-prop-1-enyl]phenol | |
| Other names
2-(1-Propenyl)-4-methoxyphenol trans-Pseudoisoeugenol | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C10H12O2 | |
| Molar mass | 164.204 g·mol−1 |
| Density | 1.0 g/cm3 |
| Boiling point | 357.3 °C (675.1 °F; 630.5 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Natural occurrence and derivatives
Pseudoisoeugenol naturally occurs in the essential oils of roots from plants within the genus Pimpinella.[1][2] In addition to its standard form, the compound also occurs in a variety of structural derivatives. Common derivatives include the compound with its side chain bearing an epoxide functional group and the aromatic ring being associated with one of many possible esters in the 2nd position. Common esters include angelic acid, 2-methylbutanoic acid, tiglic acid, and 2-methylpropionic acid esters.[1] Hydrolysis of these esters, either in-vivo or by using strong acids, forms 2-methyl-5-methoxybenzofuran.[3]
Biosynthesis
Biosynthesis of the compound is hypothesized to proceed via a NIH shift of anethole.[4]
See also
References
- Reichling, Jürgen; Galati, Enza Maria (2004). Jodral, Manuel Miró (ed.). Illicium, Pimpinella and Foeniculum (1st ed.). New York: CRC Press LLC. pp. 79–90. ISBN 9780203022320.
- Santos, Paula M.; Figueiredo, A. Cristina; Oliveira, M. Margarida; Barroso, José; Pedro, Luis G.; Deans, Stanley G.; Younus, A.K.M.; Scheffer, Johannes J.C. (1998). "Essential oils from hairy root cultures and from fruits and roots of Pimpinella anisum". Phytochemistry. 48 (3): 455–460. doi:10.1016/S0031-9422(98)00022-3.
- Martin, R.; Reichling, J.; Becker, H. (1985). "Reinvestigation of the Phenylpropanoids from the Roots of Pimpinella Species". Planta Medica. 51 (3): 198–202. doi:10.1055/s-2007-969455.
- Reichling, Jürgen; Martin, Rainer (1990). "Further Studies on the Biosynthesis of Pseudoisoeugenols in Tissue Cultures of Pimpinella anisum". Institut für Pharmazeutische Biologie der Universität Heidelberg. 45 (9–10): 942–948.