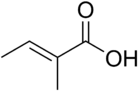
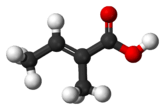
Tiglic acid
Tiglic acid is a monocarboxylic unsaturated organic acid. It is found in croton oil and in several other natural products. It was also isolated from the defensive secretion of certain beetles.[1]
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
(2E)-2-Methylbut-2-enoic acid | |
| Other names
(E)-2-Methylbut-2-enoic acid Cevadic acid Sabadillic acid Tiglinic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.001.178 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C5H8O2 | |
| Molar mass | 100.116 g/mol |
| Density | 0.9641 g/cm3 (76 °C) |
| Melting point | 63.5 to 64 °C (146.3 to 147.2 °F; 336.6 to 337.1 K) |
| Boiling point | 198.5 °C (389.3 °F; 471.6 K) |
| Acidity (pKa) | 4.96 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Properties and uses
Tiglic acid has a double bond between the second and third carbons of the chain. Tiglic acid and angelic acid form a pair of cis-trans isomers. Tiglic acid is a volatile and crystallizable substance with a sweet, warm, spicy odour. It is used in making perfumes and flavoring agents. The salts and esters of tiglic acid are called tiglates.
Toxicity
Tiglic acid is a skin and eye irritant. The inhalation of the substance causes respiratory tract irritation. It is listed on the Toxic Substances Control Act (TSCA).
Names and discovery
In 1819 Pelletier and Caventou isolated a peculiar volatile and crystallizable acid from the seeds of Schoenocaulon officinalis, a Mexican plant of family Melanthaceae (also called cevadilla or sabadilla). Consequently, the substance was named sabadillic or cevadic acid. It was later found to be identical with Frankland and Duppa's methylcrotonic acid (1865). In 1870 Geuther and Fröhlich prepared an acid from croton oil to which they gave the name tiglic acid (or tiglinic acid) after Croton tiglium (Linn.), specific name of the croton oil plant.[2] The compound was shown to be identical with the previously described methyl-crotonic acid.
See also
- Tiglyl-CoA, a thioester with coenzyme A
References
- Attygalle, A. B.; Wu, X.; Will, K. W. (2007). "Biosynthesis of tiglic, ethacrylic, and 2-methylbutyric acids in a carabid beetle, Pterostichus (Hypherpes) californicus". J Chem Ecol. 33: 963–970. PMID 17404818.
- Lloyd, J. U. (1898). "Croton tiglium Archived 2003-08-01 at the Wayback Machine". Lloyd Brothers plant drug pamphlets, Lloyd Brothers Pharmacy: Cincinnati.