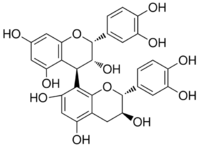
Procyanidin B2
Procyanidin B2 is a B type proanthocyanidin. Its structure is (−)-Epicatechin-(4β→8)-(−)-epicatechin.
 | |
| Names | |
|---|---|
| IUPAC name
(2R,2ʼR,3R,3ʼR,4R)-2,2ʼ-bis(3,4-dihydroxyphenyl)-3,3ʼ,4,4ʼ-tetrahydro-2H,2ʼH-4,8ʼ-bichromene-3,3ʼ,5,5ʼ,7,7ʼ-hexol | |
| Other names
Procyanidin-B2 (−)-Epicatechin-(4β→8)-(−)-epicatechin | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C30H26O12 | |
| Molar mass | 578.52 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Procyanidin B2 can be found in Cinchona pubescens (Chinchona: in the rind, bark, and cortex), in Cinnamomum verum (Ceylon cinnamon: in the rind, bark, and cortex), in Crataegus monogyna (Common hawthorn: in the flower and blossom), in Uncaria guianensis (Cat's claw: in the root), in Vitis vinifera (Common grape vine: in the leaf),[1] in Litchi chinensis (litchi: in the pericarp),[2] in the apple,[3] and in Ecdysanthera utilis.[4]
Procyanidin B2 can be converted into procyanidin A2 by radical oxidation using 1,1-diphenyl-2-picrylhydrazyl (DPPH) radicals under neutral conditions.[5]
Procyanidin B2 has been shown to inhibit the formation of the advanced glycation end-products pentosidine, carboxymethyllysine (CML), and methylglyoxal (MGO).[6]
See also
References
- Proanthocyanidin-B2 on liberherbarum.com
- Immunomodulatory and anticancer activities of flavonoids extracted from litchi (Litchi chinensis Sonn.) pericarp. Mouming Zhao; Bao Yang; Jinshui Wang; Yang Liu; Limei Yu; Yueming Jiang, 2007
- Proanthocyanidin-B2 on fuzing.com
- Lin LC (2002). "Immunomodulatory Proanthocyanidins from Ecdysanthera u tilis". Journal of Natural Products. 65 (4): 505–508. doi:10.1021/np010414l. PMID 11975489.
- Kondo K (2000). "Conversion of procyanidin B-type (catechin dimer) to A-type: evidence for abstraction of C-2 hydrogen in catechin during radical oxidation". Tetrahedron Letters. 41: 485–488. doi:10.1016/S0040-4039(99)02097-3.
- Peng X, Ma J, Chao J, Sun Z, Chang RC, Tse I, Li ET, Chen F, Wang M (2010). "Beneficial effects of cinnamon proanthocyanidins on the formation of specific advanced glycation endproducts and methylglyoxal-induced impairment on glucose consumption". Journal of Agricultural and Food Chemistry. 58 (11): 6692–6696. doi:10.1021/jf100538t. PMID 20476737.