Polycarbophil calcium
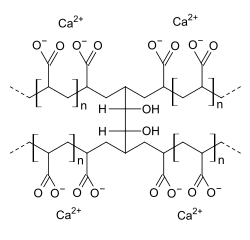
Polycarbophil calcium (INN) is a drug used as a stool stabilizer. Chemically, it is a synthetic polymer of polyacrylic acid cross-linked with divinyl glycol, with calcium as a counter-ion.
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Multum Consumer Information |
| Pregnancy category |
|
| Routes of administration | Oral (tablets) |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number | |
| PubChem SID | |
| ChemSpider |
|
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | (C3H3Ca1/2O2)a(C6H10O2)b |
| | |
Clinical uses
It is used as stool stabilizer to treat constipation, diarrhea and abdominal discomfort. Bulk laxatives absorb liquid in the intestines and swell to form a soft bulky stool. The bulky mass stimulates the intestinal muscles, speeding stool transit time through the colon. Results usually occur within 12 to 72 hours. Calcium polycarbophil will not work without increased fluid intake.
Calcium polycarbophil has been marketed as an over-the-counter agent used for treating functional bowel disorder and as a bulk-producing agent.
A study looked at the effects of calcium polycarbophil on general irritable bowel syndrome (IBS) symptoms. Fourteen patients with IBS-diarrhea and twelve with IBS-constipation were given calcium polycarbophil for eight weeks and their colon transit times were measured with radiopaque markers in the colon. The patients with diarrhea reported fewer bowel movements, more solid stools and reduced abdominal pain. Patients with constipation reported more frequent bowel movements, looser stools and less pain.[1]
The human stomach presents a mild acidic environment due to the presence of HCl. Polycarbophil absorbs about ten times its own weight of water under acidic conditions, but the swelling ratio markedly increases at above pH 4.0 and reaches 70 times the initial weight under pH-neutral conditions. The swelling of polycarbophil is not affected by non-ionic osmolarity, but by ionic strength, showing a decrease with increase of ionic strength. Monovalent metal ions such as sodium and potassium ions in gastrointestinal fluid do not reduce the equilibrium swelling of polycarbophil, but divalent ions such as calcium and magnesium ions do. However, calcium ions only slightly reduce the equilibrium swelling under sodium-rich conditions.[2]
References
- Chiba T, Kudara N, Sato M, Chishima R, Abiko Y, Inomata M, et al. (2005). "Colonic transit, bowel movements, stool form, and abdominal pain in irritable bowel syndrome by treatments with calcium polycarbophil". Hepato-Gastroenterology. 52 (65): 1416–20. PMID 16201086.
- Shibata C, Funayama Y, Fukushima K, Takahashi K, Ogawa H, Haneda S, et al. (June 2007). "Effect of calcium polycarbophil on bowel function after restorative proctocolectomy for ulcerative colitis: a randomized controlled trial". Digestive Diseases and Sciences. 52 (6): 1423–6. doi:10.1007/s10620-006-9270-6. PMID 17394081.
Further reading
- Saito T, Mizutani F, Iwanaga Y, Morikawa K, Kato H (June 2002). "Laxative and anti-diarrheal activity of polycarbophil in mice and rats". Japanese Journal of Pharmacology. 89 (2): 133–41. doi:10.1254/jjp.89.133. PMID 12120755.
- Torii A, Toda G (May 2004). "Management of irritable bowel syndrome". Internal Medicine. 43 (5): 353–9. doi:10.2169/internalmedicine.43.353. PMID 15206545.
- Paterson WG, Thompson WG, Vanner SJ, Faloon TR, Rosser WW, Birtwhistle RW, et al. (July 1999). "Recommendations for the management of irritable bowel syndrome in family practice. IBS Consensus Conference Participants". CMAJ. 161 (2): 154–60. PMC 1230466. PMID 10439825.