Podosome
Podosomes are conical, actin-rich structures found on the outer surface of the plasma membrane of animal cells.[1] Their size ranges from approximately 0.5 µm to 2.0 µm in diameter. While usually situated on the periphery of the cellular membrane, these unique structures display a polarized pattern of distribution in migrating cells, situating at the front border between the lamellipodium and lamellum.[2] Their primary purpose is connected to cellular motility and invasion; therefore, they serve as both sites of attachment and degradation along the extracellular matrix. Many different specialized cells exhibit these dynamic structures such as invasive cancer cells, osteoclasts, vascular smooth muscle cells, endothelial cells, and certain immune cells like macrophages and dendritic cells.[3]
| Podosome | |
|---|---|
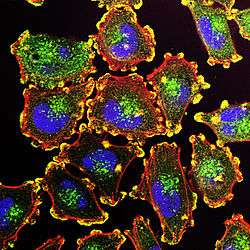 Podosomes (yellow) in melanoma cells, along with cell nuclei (blue), actin (red), and an actin regulator (green). | |
| Details | |
| Identifiers | |
| Latin | Podosoma |
| MeSH | D000069261 |
| TH | H1.00.01.1.02034 |
| Anatomical terminology | |
Characteristics
A podosome consists of a core rich in actin surrounded by adhesion and scaffolding proteins. The actin filaments within these structures are highly regulated by many actin nucleators, polymerization activators, actin binding and crosslinking proteins, kinases, small GTPases, and scaffold proteins; therefore, total actin turnover occurs within seconds.[4] To distinguish podosomes from others types of cellular adhesions, the protein Tks5 and WASP (Wiskott–Aldrich syndrome protein) are used as markers alongside actin, cortactin and the Arp2/3 complex to localize and isolate these protrusions because Tks5 and WASP are unique to the podosome when compared with other actin-based cellular structures.[5]
In their outward structure, the podosomes demonstrate two distinct features: an actin core and a ring complex. Within the core, coordinators of actin nucleation are found. Specifically, the Arp2/3 complex and WASP when close to the plasma membrane or cortactin when further away comprise this group of proteins. Emanating radially from the dense core of actin are actin filaments reaching to the plasma membrane and between neighboring podosomes.[6]
In the ring complex, integrins and integrin-associated proteins serve to connect the cytoskeleton to cell surface integrins forming the outward protrusion.[7] Initial research suggested that the superstructure of podosomes were cylindrical, but new advances in bioimaging techniques have altered that perception and show the ring complex to display a polygonal form. These finding were made possible through the application of Bayesian blinking and bleaching analytics to data gained from standard widefield microscopy using cells that expressed fluorescently tagged proteins specific to the podosome ring complex.[8]
Typically, the podosome size falls between 0.5 um and 2.0 um in diameter and depth. The lifetime of the structure is only minutes in duration, much shorter than observed in invadopodia.[9][10]
Function
Podosomes are thought to be intimately connected to cellular motility within tissue microenvironments through coordinating degradation of the extracellular matrix with cellular movement. The migration of cells is essential to proper embryonic development and, in maturity, to wound healing and the inflammatory response.[11] Examples of these motile cell behaviors include: transendothelial migration of dendritic cells, migration of aortic endothelial cells for arterial vessel remodeling, and tissue infiltration by macrophages. Aberrations in cell migration lie beneath pathologies involving development, vasculature, and immunity. Consequently, podosomes are present in cell types associated with tissue remodeling and the immune system.[12][13]
Patients who suffer from Wiskott–Aldrich syndrome demonstrate, through their immune cells, continued evidence of the role podosomes fulfill in cell motility. These patients do not possess fully formed WASP that has been shown to localize in podosomes and to be integral to their formation from previous studies.[14] The dendritic cells and macrophages of these patients’ immune systems do not manifest podosome formations and demonstrate defects in cellular movement within tissue microenvironments.[15] Some researchers suspect that podosomes may be implicated in the migration of neural crest cells. Patients who exhibit Frank–ter Haar syndrome are known to be mutant for the podosome specific protein Tks4 and demonstrate defects in neural crest cell migration.[16]
Adding to the known functionalities of podosomes, research suggests that these dynamic structures also exhibit mechanosensory attributes.[17] Initial formation of podosomes seems to be influenced by the structure and composition of the underlying substratum including the presence and distribution of specific ligands.[18] Various integrin receptors monitor the mechanical properties of the cellular microenvironment and can influence and initiate formation of a podosome. Once fully formed, the integrity of the matrix substratum dictates the lifespan of the podosome with increased stiffness leading to longer endurance and closer spacing between podosome sites.[19]
Some studies indicates also a putative role for podosomes even in the regulation of bone marrow stem cell's function. Podosomes have been shown to be widely present in vitro on mesodermal progenitor cells (MPCs), cell capable of differentiating into mesenchymal stromal cells. It has been proposed that podosomes are important in the mobilisation of MPCs in the event of physiological need.[20]
Role in osteoclasts
Osteoclasts are large, multinucleated bone cells that conduct the process of bone resorption. In this remodeling process, podosomes play an integral role.[21] During the maturation of osteoclast precursors, groups of podosomes form higher ordered ring structures which ultimately coalesce into a band about the cell periphery. The resulting arrangement of podosomes is highly interconnected through a dense, radial network of actin filaments that extend between and onto neighboring podosomes.[22]
Accumulation of F-actin, vinculin, paxillin, and α-actin within the podosomes of the coalescent band signals the development of a fully matured osteoclast.[23] Upon initiation of bone resorption, the band of podosomes disassembles leaving behind a mesh primarily composed of F-actin which functions as the ‘sealing zone.’ This sealing zone becomes the site of osteoclast attachment to the bone matrix.[24] Inhibition of bone resorption through drug intervention results in the lack of the podosome band during early osteoclast differentiation and ultimate absence of a sealing zone.[25]
History
In the early 1980s, chicken embryo fibroblasts were transformed using the Rous sarcoma virus (RSV) containing the oncogene v-src. This transformation elicited the relocalization of vinculin and α-actin in the cytoskeleton from focal adhesions forming circular clusters. Later in 1985, it was shown using the same cells that these protein clusters were localized to protrusions in the ventral plasma membrane, were substratum adhesion sites; therefore, these structures were termed podosomes indicating their foot-like character in cells. In 1989, it was demonstrated that these podosomes played a role in matrix degradation. To reflect this newly discovered destructive nature the name invadopodia was given to these dynamic structures.[26]
Because both terms invadopodia and podosomes were initially used to reference the identical structures in identical cell lines, there exists confusion about the nomenclature. Typically, when these structures are found in normal cells, they are referred to as podosomes, and when in cancer cells, invadopodia.
See also
References
- Rottiers, P; Saltel, F; Daubon, T; Chaigne-Delalande, B; Tridon, V; Billottet, C; Reuzeau, E; Génot, E (Dec 1, 2009). "TGFbeta-induced endothelial podosomes mediate basement membrane collagen degradation in arterial vessels". Journal of Cell Science. 122 (Pt 23): 4311–8. doi:10.1242/jcs.057448. PMID 19887587.
- Calle, Y; Burns, S; Thrasher, AJ; Jones, GE (April 2006). "The leukocyte podosome". European Journal of Cell Biology. 85 (3–4): 151–7. doi:10.1016/j.ejcb.2005.09.003. PMID 16546557.
- Gimona, M; Buccione, R; Courtneidge, SA; Linder, S (April 2008). "Assembly and biological role of podosomes and invadopodia". Current Opinion in Cell Biology. 20 (2): 235–41. doi:10.1016/j.ceb.2008.01.005. PMID 18337078.
- Calle, Y; Chou, HC; Thrasher, AJ; Jones, GE (November 2004). "Wiskott–Aldrich syndrome protein and the cytoskeletal dynamics of dendritic cells". The Journal of Pathology. 204 (4): 460–9. doi:10.1002/path.1651. PMID 15495215.
- Morton, PE; Parsons, M (Jul–Aug 2011). "Dissecting cell adhesion architecture using advanced imaging techniques". Cell Adhesion & Migration. 5 (4): 351–9. doi:10.4161/cam.5.4.16915. PMC 3210303. PMID 21785274.
- Akisaka, T; Yoshida, H; Suzuki, R; Takama, K (March 2008). "Adhesion structures and their cytoskeleton-membrane interactions at podosomes of osteoclasts in culture". Cell and Tissue Research. 331 (3): 625–41. doi:10.1007/s00441-007-0552-x. PMID 18087726.
- Linder, S (March 2007). "The matrix corroded: podosomes and invadopodia in extracellular matrix degradation". Trends in Cell Biology. 17 (3): 107–17. doi:10.1016/j.tcb.2007.01.002. PMID 17275303.
- Cox, S; Rosten, E; Monypenny, J; Jovanovic-Talisman, T; Burnette, DT; Lippincott-Schwartz, J; Jones, GE; Heintzmann, R (Dec 4, 2011). "Bayesian localization microscopy reveals nanoscale podosome dynamics". Nature Methods. 9 (2): 195–200. doi:10.1038/nmeth.1812. PMC 3272474. PMID 22138825.
- Cox, S; Rosten, E; Monypenny, J; Jovanovic-Talisman, T; Burnette, DT; Lippincott-Schwartz, J; Jones, GE; Heintzmann, R (Dec 4, 2011). "Bayesian localization microscopy reveals nanoscale podosome dynamics". Nature Methods. 9 (2): 195–200. doi:10.1038/nmeth.1812. PMC 3272474. PMID 22138825.
- Sharma, Ved P.; Eddy, Robert; Entenberg, David; Kai, Masayuki; Gertler, Frank B.; Condeelis, John (2013-11-04). "Tks5 and SHIP2 regulate invadopodium maturation, but not initiation, in breast carcinoma cells". Current Biology. 23 (21): 2079–2089. doi:10.1016/j.cub.2013.08.044. ISSN 1879-0445. PMC 3882144. PMID 24206842.
- Murphy, DA; Courtneidge, SA (Jun 23, 2011). "The 'ins' and 'outs' of podosomes and invadopodia: characteristics, formation and function". Nature Reviews. Molecular Cell Biology. 12 (7): 413–26. doi:10.1038/nrm3141. PMC 3423958. PMID 21697900.
- Calle, Y; Carragher, NO; Thrasher, AJ; Jones, GE (Jun 1, 2006). "Inhibition of calpain stabilises podosomes and impairs dendritic cell motility". Journal of Cell Science. 119 (Pt 11): 2375–85. doi:10.1242/jcs.02939. PMID 16723743.
- Cougoule, C; Le Cabec, V; Poincloux, R; Al Saati, T; Mège, J. L.; Tabouret, G; Lowell, C. A.; Laviolette-Malirat, N; Maridonneau-Parini, I (Feb 18, 2010). "Three-dimensional migration of macrophages requires Hck for podosome organization and extracellular matrix proteolysis". Blood. 115 (7): 1444–52. doi:10.1182/blood-2009-04-218735. PMC 5070714. PMID 19897576.
- Burns, S; Thrasher, AJ; Blundell, MP; Machesky, L; Jones, GE (Aug 15, 2001). "Configuration of human dendritic cell cytoskeleton by Rho GTPases, the WAS protein, and differentiation". Blood. 98 (4): 1142–9. doi:10.1182/blood.v98.4.1142. PMID 11493463.
- Linder, S; Nelson, D; Weiss, M; Aepfelbacher, M (Aug 17, 1999). "Wiskott-Aldrich syndrome protein regulates podosomes in primary human macrophages". Proceedings of the National Academy of Sciences of the United States of America. 96 (17): 9648–53. doi:10.1073/pnas.96.17.9648. PMC 22264. PMID 10449748.
- Murphy, DA; Courtneidge, SA (Jun 23, 2011). "The 'ins' and 'outs' of podosomes and invadopodia: characteristics, formation and function". Nature Reviews. Molecular Cell Biology. 12 (7): 413–26. doi:10.1038/nrm3141. PMC 3423958. PMID 21697900.
- Labernadie, A; Thibault, C; Vieu, C; Maridonneau-Parini, I; Charrière, GM (Dec 7, 2010). "Dynamics of podosome stiffness revealed by atomic force microscopy". Proceedings of the National Academy of Sciences of the United States of America. 107 (49): 21016–21. doi:10.1073/pnas.1007835107. PMC 3000246. PMID 21081699.
- Linder, S; Wiesner, C; Himmel, M (Nov 10, 2011). "Degrading devices: invadosomes in proteolytic cell invasion". Annual Review of Cell and Developmental Biology. 27: 185–211. doi:10.1146/annurev-cellbio-092910-154216. PMID 21801014.
- Collin, O; Tracqui, P; Stephanou, A; Usson, Y; Clément-Lacroix, J; Planus, E (May 1, 2006). "Spatiotemporal dynamics of actin-rich adhesion microdomains: influence of substrate flexibility". Journal of Cell Science. 119 (Pt 9): 1914–25. doi:10.1242/jcs.02838. PMID 16636076.
- Pacini, S, O; Fazzi, R; Montali, M; Carnicelli, V; Lazzarini, E; Petrini, M (Jun 15, 2013). "Specific integrin expression is associated with podosome-like structures on mesodermal progenitor cells". Stem Cells and Development. 22 (Pt 12): 1830–38. doi:10.1089/scd.2012.0423. PMID 23379672.
- Destaing, O; Saltel, F; Géminard, JC; Jurdic, P; Bard, F (February 2003). "Podosomes display actin turnover and dynamic self-organization in osteoclasts expressing actin-green fluorescent protein". Molecular Biology of the Cell. 14 (2): 407–16. doi:10.1091/mbc.E02-07-0389. PMC 149981. PMID 12589043.
- Luxenburg, C; Geblinger, D; Klein, E; Anderson, K; Hanein, D; Geiger, B; Addadi, L (Jan 31, 2007). "The architecture of the adhesive apparatus of cultured osteoclasts: from podosome formation to sealing zone assembly". PLoS ONE. 2 (1): e179. doi:10.1371/journal.pone.0000179. PMC 1779809. PMID 17264882.

- Luxenburg, C; Addadi, L; Geiger, B (April 2006). "The molecular dynamics of osteoclast adhesions". European Journal of Cell Biology. 85 (3–4): 203–11. doi:10.1016/j.ejcb.2005.11.002. PMID 16360241.
- Luxenburg, C; Parsons, JT; Addadi, L; Geiger, B (Dec 1, 2006). "Involvement of the Src-cortactin pathway in podosome formation and turnover during polarization of cultured osteoclasts". Journal of Cell Science. 119 (Pt 23): 4878–88. doi:10.1242/jcs.03271. PMID 17105771.
- Ishida, T; Fujiwara, K (February 1979). "Pathology of diarrhea due to mouse hepatitis virus in the infant mouse". The Japanese Journal of Experimental Medicine. 49 (1): 33–41. PMID 224229.
- Murphy, DA; Courtneidge, SA (Jun 23, 2011). "The 'ins' and 'outs' of podosomes and invadopodia: characteristics, formation and function". Nature Reviews. Molecular Cell Biology. 12 (7): 413–26. doi:10.1038/nrm3141. PMC 3423958. PMID 21697900.
External links
- MBInfo - Podosomes
- MBInfo - Podosome Assembly
- Podosomes and Invadopodia at Scirus Topic Pages