Streptococcus pneumoniae
Streptococcus pneumoniae, or pneumococcus, is a Gram-positive, spherical bacteria, alpha-hemolytic (under aerobic conditions) or beta-hemolytic (under anaerobic conditions), facultative anaerobic member of the genus Streptococcus.[1] They are usually found in pairs (diplococci) and do not form spores and are non motile.[2] As a significant human pathogenic bacterium S. pneumoniae was recognized as a major cause of pneumonia in the late 19th century, and is the subject of many humoral immunity studies.
| Streptococcus pneumoniae | |
|---|---|
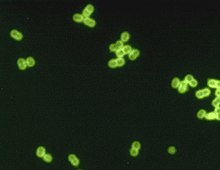 | |
| S. pneumoniae in spinal fluid. FA stain (digitally colored). | |
| Scientific classification | |
| Domain: | Bacteria |
| Phylum: | Firmicutes |
| Class: | Bacilli |
| Order: | Lactobacillales |
| Family: | Streptococcaceae |
| Genus: | Streptococcus |
| Species: | S. pneumoniae |
| Binomial name | |
| Streptococcus pneumoniae (Klein 1884) Chester 1901 | |
Streptococcus pneumoniae resides asymptomatically in healthy carriers typically colonizing the respiratory tract, sinuses, and nasal cavity. However, in susceptible individuals with weaker immune systems, such as the elderly and young children, the bacterium may become pathogenic and spread to other locations to cause disease. It spreads by direct person-to-person contact via respiratory droplets and by auto inoculation in persons carrying the bacteria in their upper respiratory tracts.[3] It can be a cause of neonatal infections.[4]
Streptococcus pneumoniae is the main cause of community acquired pneumonia and meningitis in children and the elderly,[5] and of sepsis in those infected with HIV. The organism also causes many types of pneumococcal infections other than pneumonia. These invasive pneumococcal diseases include bronchitis, rhinitis, acute sinusitis, otitis media, conjunctivitis, meningitis, sepsis, osteomyelitis, septic arthritis, endocarditis, peritonitis, pericarditis, cellulitis, and brain abscess.[6]
Streptococcus pneumoniae can be differentiated from the viridans streptococci, some of which are also alpha-hemolytic, using an optochin test, as S. pneumoniae is optochin-sensitive. S. pneumoniae can also be distinguished based on its sensitivity to lysis by bile, the so-called "bile solubility test". The encapsulated, Gram-positive, coccoid bacteria have a distinctive morphology on Gram stain, lancet-shaped diplococci. They have a polysaccharide capsule that acts as a virulence factor for the organism; more than 90 different serotypes are known, and these types differ in virulence, prevalence, and extent of drug resistance.
History
In 1881, the organism, known later in 1886 as the pneumococcus[7] for its role as a cause of pneumonia, was first isolated simultaneously and independently by the U.S. Army physician George Sternberg[8] and the French chemist Louis Pasteur.[9]
The organism was termed Diplococcus pneumoniae from 1920[10] because of its characteristic appearance in Gram-stained sputum. It was renamed Streptococcus pneumoniae in 1974 because it was very similar to streptococci.[7][11]
Streptococcus pneumoniae played a central role in demonstrating that genetic material consists of DNA. In 1928, Frederick Griffith demonstrated transformation of life turning harmless pneumococcus into a lethal form by co-inoculating the live pneumococci into a mouse along with heat-killed virulent pneumococci.[12] In 1944, Oswald Avery, Colin MacLeod, and Maclyn McCarty demonstrated that the transforming factor in Griffith's experiment was not protein, as was widely believed at the time, but DNA.[13] Avery's work marked the birth of the molecular era of genetics.[14]
Genetics
The genome of S. pneumoniae is a closed, circular DNA structure that contains between 2.0 and 2.1 million base pairs depending on the strain. It has a core set of 1553 genes, plus 154 genes in its virulome, which contribute to virulence and 176 genes that maintain a noninvasive phenotype. Genetic information can vary up to 10% between strains.[15] The pneumococcal genome is known to contain a large and diverse repertoire of antimicrobial peptides, including 11 different lantibiotics.[16]
Transformation
Natural bacterial transformation involves the transfer of DNA from one bacterium to another through the surrounding medium. Transformation is a complex developmental process requiring energy and is dependent on expression of numerous genes. In S. pneumoniae, at least 23 genes are required for transformation. For a bacterium to bind, take up, and recombine exogenous DNA into its chromosome, it must enter a special physiological state called competence.
Competence in S. pneumoniae is induced by DNA-damaging agents such as mitomycin C, fluoroquinolone antibiotics (norfloxacin, levofloxacin and moxifloxacin), and topoisomerase inhibitors.[17] Transformation protects S. pneumoniae against the bactericidal effect of mitomycin C.[18] Michod et al.[19] summarized evidence that induction of competence in S. pneumoniae is associated with increased resistance to oxidative stress and increased expression of the RecA protein, a key component of the recombinational repair machinery for removing DNA damages. On the basis of these findings, they suggested that transformation is an adaptation for repairing oxidative DNA damages. S. pneumoniae infection stimulates polymorphonuclear leukocytes (granulocytes) to produce an oxidative burst that is potentially lethal to the bacteria. The ability of S. pneumoniae to repair the oxidative DNA damages in its genome, caused by this host defense, likely contributes to this pathogen's virulence. Consistent with this premise, Li et al.[20] reported that, among different highly transformable S. pneumoniae isolates, nasal colonization fitness and virulence (lung infectivity) depend on an intact competence system.
Infection
Streptococcus pneumoniaeis part of the normal upper respiratory tract flora. As with many natural flora, it can become pathogenic under the right conditions, typically when the immune system of the host is suppressed. Invasins, such as pneumolysin, an antiphagocytic capsule, various adhesins, and immunogenic cell wall components are all major virulence factors. After S. pneumoniae colonizes the air sacs of the lungs, the body responds by stimulating the inflammatory response, causing plasma, blood, and white blood cells to fill the alveoli. This condition is called pneumonia.[21]
Diseases and symptoms
Pneumonia is the most common of the S. pneumoniae diseases which include symptoms such as fever and chills, cough, rapid breathing, difficulty breathing, and chest pain. For the elderly, they may include confusion, low alertness, and the former listed symptoms to a lesser degree.
Pneumococcal meningitis is an infection of the tissue covering the brain and spinal cord. Symptoms include stiff neck, fever, headache, confusion, and photophobia.
Sepsis is caused by overwhelming response to an infection and leads to tissue damage, organ failure, and even death. The symptoms include confusion, shortness of breath, elevated heart rate, pain or discomfort, over-perspiration, fever, shivering, or feeling cold.[22]
Vaccine
Due to the importance of disease caused by S. pneumoniae, several vaccines have been developed to protect against invasive infection. The World Health Organization recommends routine childhood pneumococcal vaccination;[23] it is incorporated into the childhood immunization schedule in a number of countries including the United Kingdom,[24] the United States,[25] and South Africa.[26]
Interaction with Haemophilus influenzae
Historically, Haemophilus influenzae has been a significant cause of infection, and both H. influenzae and S. pneumoniae can be found in the human upper respiratory system. A study of competition in vitro revealed S. pneumoniae overpowered H. influenzae by attacking it with hydrogen peroxide.[27] However, in a study adding both bacteria to the nasal cavity of a mouse within 2 weeks, only H. influenzae survives; further analysis showed that neutrophils exposed to dead H. influenzae were more aggressive in attacking S. pneumoniae.[28]
Diagnosis
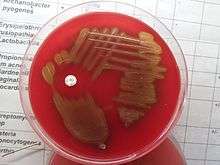
Diagnosis is generally made based on clinical suspicion along with a positive culture from a sample from virtually any place in the body. S. pneumoniae is, in general, optochin sensitive, although optochin resistance has been observed.[29]
The recent advances in next-generation sequencing and comparative genomics have enabled the development of robust and reliable molecular methods for the detection and identification of S. pneumoniae. For instance, the Xisco gene was recently described as a biomarker for PCR-based detection of S. pneumoniae and differentiation from closely related species.[30]
Atromentin and leucomelone possess antibacterial activity, inhibiting the enzyme enoyl-acyl carrier protein reductase, (essential for the biosynthesis of fatty acids) in S. pneumoniae.[31]
Resistance
Resistant pneumococcal strains are called penicillin-resistant pneumococci (PRP),[32] penicillin-resistant Streptococcus pneumoniae (PRSP),[33] Streptococcus pneumoniae penicillin resistant (SPPR)[34] or drug-resistant Strepotococcus pneumoniae (DRSP). In 2015, in the US, there were an estimated 30,000 cases, and in 30% of them the strains were resistant to one or more antibiotics.[35]
References
- Ryan KJ, Ray CG, eds. (2004). Sherris Medical Microbiology. McGraw Hill. ISBN 978-0-8385-8529-0.
- "Streptococcus pneumoniae". microbewiki.kenyon.edu. Retrieved 2017-10-24.
- "Transmission". cdc.org. Retrieved 24 Oct 2017.
- Baucells, B.J.; Mercadal Hally, M.; Álvarez Sánchez, A.T.; Figueras Aloy, J. (2015). "Asociaciones de probióticos para la prevención de la enterocolitis necrosante y la reducción de la sepsis tardía y la mortalidad neonatal en recién nacidos pretérmino de menos de 1.500g: una revisión sistemática". Anales de Pediatría. 85 (5): 247–255. doi:10.1016/j.anpedi.2015.07.038. ISSN 1695-4033. PMID 26611880.
- van de Beek, Diederik; de Gans, Jan; Tunkel, Allan R.; Wijdicks, Eelco F.M. (5 January 2006). "Community-Acquired Bacterial Meningitis in Adults". New England Journal of Medicine. 354 (1): 44–53. doi:10.1056/NEJMra052116. ISSN 0028-4793. PMID 16394301.
- Siemieniuk, Reed A.C.; Gregson, Dan B.; Gill, M. John (Nov 2011). "The persisting burden of invasive pneumococcal disease in HIV patients: an observational cohort study". BMC Infectious Diseases. 11: 314. doi:10.1186/1471-2334-11-314. PMC 3226630. PMID 22078162.
- Plotkin, Stanley; Orenstein, W; Offit, PA (September 22, 2012). Vaccines. Elsevier – Saunders. p. 542. ISBN 978-1455700905. Retrieved July 2, 2015.
- Sternberg, George Miller (30 April 1881). "A fatal form of septicaemia in the rabbit produced by the subcutaneous injection of human saliva. An experimental research". Bulletin of the National Board of Health..
- Pasteur, Louis (1881). "Sur une maladie nouvelle provoquée par la salive d'un enfant mort de rage". Comptes Rendus de l'Académie des Sciences de Paris. 92: 159..
- Winslow, C.; J. Broadhurst (1920). "The Families and Genera of the Bacteria: Final Report of the Committee of the Society of American Bacteriologists on Characterization and Classification of Bacterial Types". J Bacteriol. 5 (3): 191–229. doi:10.1128/JB.5.3.191-229.1920. PMC 378870. PMID 16558872.
- Wainer H (2014). Medical Illuminations: Using Evidence, Visualization and Statistical Thinking to Improve Healthcare. Oxford University Press. p. 53. ISBN 978-0199668793. Retrieved July 4, 2015.
- Griffith F (January 1928). "The Significance of Pneumococcal Types". Journal of Hygiene. 27 (2): 113–159. doi:10.1017/S0022172400031879. PMC 2167760. PMID 20474956.
- Avery OT, MacLeod CM, McCarty M (1944). "Studies on the chemical nature of the substance inducing transformation of pneumococcal types: induction of transformation by a desoxyribonucleic acid fraction isolated from pneumococcus type III". J Exp Med. 79 (2): 137–158. doi:10.1084/jem.79.2.137. PMC 2135445. PMID 19871359.
- Lederberg J (1994). "The Transformation of Genetics by DNA: An Anniversary Celebration of Avery, Macleod and Mccarty (1944)". Genetics. 136 (2): 423–6. PMC 1205797. PMID 8150273.
- van der Poll T, Opal SM (2009). "Pathogenesis, treatment, and prevention of pneumococcal pneumonia". Lancet. 374 (9700): 1543–56. doi:10.1016/S0140-6736(09)61114-4. PMID 19880020.
- Rezaei Javan, Reza; Van Tonder, Andries; King, James; Harrold, Caroline; Brueggemann, Angela (August 2018). "Genome Sequencing Reveals a Large and Diverse Repertoire of Antimicrobial Peptides". Frontiers in Microbiology. 2012 (9): 2012. doi:10.3389/fmicb.2018.02012. PMC 6120550. PMID 30210481.
- Claverys JP, Prudhomme M, Martin B (2006). "Induction of competence regulons as a general response to stress in gram-positive bacteria". Annu. Rev. Microbiol. 60: 451–75. doi:10.1146/annurev.micro.60.080805.142139. PMID 16771651.
- Engelmoer DJ, Rozen DE (December 2011). "Competence increases survival during stress in Streptococcus pneumoniae". Evolution. 65 (12): 3475–85. doi:10.1111/j.1558-5646.2011.01402.x. PMID 22133219.
- Michod RE, Bernstein H, Nedelcu AM (May 2008). "Adaptive value of sex in microbial pathogens" (PDF). Infect. Genet. Evol. 8 (3): 267–85. doi:10.1016/j.meegid.2008.01.002. PMID 18295550.
- Li G, Liang Z, Wang X, Yang Y, Shao Z, Li M, Ma Y, Qu F, Morrison DA, Zhang JR (2016). "Addiction of Hypertransformable Pneumococcal Isolates to Natural Transformation for In Vivo Fitness and Virulence". Infect. Immun. 84 (6): 1887–901. doi:10.1128/IAI.00097-16. PMC 4907133. PMID 27068094.
- Anderson, Cindy. "Pathogenic Properties (Virulence Factors) of Some Common Pathogens" (PDF).
- "Symptoms and Complications". Centers for Disease Control and Prevention.
- "Pneumococcal vaccines WHO position paper--2012" (PDF). Wkly Epidemiol Rec. 87 (14): 129–44. Apr 6, 2012. PMID 24340399.
- "Children to be given new vaccine". BBC News. 8 February 2006.
- "Pneumococcal Vaccination: Information for Health Care Providers". cdc.org. Archived from the original on 23 July 2016. Retrieved 26 July 2016.
- "Critical decline in pneumococcal disease and antibiotic resistance in South Africa". NICD. Retrieved 20 July 2015.
- Pericone, Christopher D.; Overweg, Karin; Hermans, Peter W. M.; Weiser, Jeffrey N. (2000). "Inhibitory and Bactericidal Effects of Hydrogen Peroxide Production by Streptococcus pneumoniae on Other Inhabitants of the Upper Respiratory Tract". Infect Immun. 68 (7): 3990–3997. doi:10.1128/IAI.68.7.3990-3997.2000. PMC 101678. PMID 10858213.
- Lysenko ES, Ratner AJ, Nelson AL, Weiser JN (2005). "The Role of Innate Immune Responses in the Outcome of Interspecies Competition for Colonization of Mucosal Surfaces". PLOS Pathog. 1 (1): e1. doi:10.1371/journal.ppat.0010001. PMC 1238736. PMID 16201010. Full text
- Pikis, Andreas; Campos, Joseph M.; Rodriguez, William J.; Keith, Jerry M. (2001). "Optochin Resistance in Streptococcus pneumoniae: Mechanism, Significance, and Clinical Implications". The Journal of Infectious Diseases. 184 (5): 582–90. doi:10.1086/322803. ISSN 0022-1899. JSTOR 30137322. PMID 11474432.
- Salvà-Serra, Francisco; Connolly, Gwendolyn; Moore, Edward R. B.; Gonzales-Siles, Lucia (2017-12-15). "Detection of "Xisco" gene for identification of Streptococcus pneumoniae isolates". Diagnostic Microbiology and Infectious Disease. 90 (4): 248–250. doi:10.1016/j.diagmicrobio.2017.12.003. ISSN 1879-0070. PMID 29329755.
- Zheng CJ, Sohn MJ, Kim WG (2006). "Atromentin and leucomelone, the first inhibitors specific to enoyl-ACP reductase (FabK) of Streptococcus pneumoniae". Journal of Antibiotics. 59 (12): 808–12. doi:10.1038/ja.2006.108. PMID 17323650.
- Nilsson, P; Laurell, MH (2001). "Carriage of penicillin-resistant Streptococcus pneumoniae by children in day-care centers during an intervention program in Malmo, Sweden". The Pediatric Infectious Disease Journal. 20 (12): 1144–9. doi:10.1097/00006454-200112000-00010. PMID 11740321.
- Block, SL; Harrison, CJ; Hedrick, JA; Tyler, RD; Smith, RA; Keegan, E; Chartrand, SA (1995). "Penicillin-resistant Streptococcus pneumoniae' in acute otitis media: risk factors, susceptibility patterns and antimicrobial management". The Pediatric Infectious Disease Journal. 14 (9): 751–9. doi:10.1097/00006454-199509000-00005. PMID 8559623.
- Koiuszko, S; Bialucha, A; Gospodarek, E (2007). "The drug susceptibility of penicillin-resistant Streptococcus pneumoniae". Medycyna Doswiadczalna I Mikrobiologia. 59 (4): 293–300. PMID 18416121.
- "Drug Resistance". cdc.gov. Retrieved 17 February 2019.
External links
| Wikimedia Commons has media related to Streptococcus pneumoniae. |
- GAVI Alliance
- PneumoADIP
- PATH's Vaccine Resource Library pneumococcal resources
- Centers for Disease Control and Prevention (2012). "Ch. 16: Pneumococcal Disease". In Atkinson W; Wolfe S; Hamborsky J (eds.). Epidemiology and Prevention of Vaccine-Preventable Diseases (12th ed.). Washington DC: Public Health Foundation. pp. 233–248. Archived from the original on 2017-03-10.
- Type strain of Streptococcus pneumoniae at BacDive - the Bacterial Diversity Metadatabase