Pertechnetate
The pertechnetate ion is an oxoanion with the chemical formula TcO−
4. It is often used as a convenient water-soluble source of isotopes of the radioactive element technetium (Tc). In particular it is used to carry the 99mTc isotope (half-life 6 hours) which is commonly used in nuclear medicine in several nuclear scanning procedures.
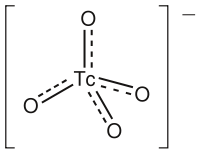
A technetate(VII) salt is a compound containing this ion. Pertechnetate compounds are salts of technetic(VII) acid. Pertechnetate is analogous to permanganate but it has little oxidizing power. Pertechnetate has higher oxidation power than perrhenate.[1]
Understanding pertechnate is important in understanding technetium contamination in the environment and in nuclear waste management.[1]
Compounds
| Formula | name | crystal structure | cell dimensions Å | unit cell volume Å3 | remarks | references |
|---|---|---|---|---|---|---|
| LiTcO4 | lithium pertechnate | [1] | ||||
| LiTcO4·2H2O | lithium pertechnate monohydrate | [1] | ||||
| LiTcO4·3H2O | lithium pertechnate trihydrate | Pt3/mc | [1] | |||
| NaTcO4 | sodium pertechnate | tetragonal | a=5.342 c=1.874 | 338.91 | absorbs water from atmosphere | [1] |
| NaTcO4·H2O | sodium pertechnate monohydrate | [1] | ||||
| NaTcO4·2H2O | sodium pertechnate dihydrate | [1] | ||||
| NaTcO4·4H2O | sodium pertechnate tetrahydrate | [1] | ||||
| KTcO4 | potassium pertechnate | tetragonal | a=5.647 c=12.91 | 411.73 | used to prepare radiopharmaceuticals | [1] |
| RbTcO4 | rubidium pertechnate | tetragonal | a=5.762 c=13.543 | 449.65 | [1] | |
| CsTcO4 | caesium pertechnate β | orthorhombic | a=5.737 b=5.92 c=14.341 | 486.38 | [1] | |
| CsTcO4 | caesium pertechnate α | tetragonal | a=5.898 c=14.38 | high temperature >470K; volatile at high temperatures | [1] | |
| TlTcO4 | thallium pertechnate | orthorhombic | [1] | |||
| TlTcO4 | thallium pertechnate | tetragonal | at high temperatures | [1] | ||
| NH4TcO4 | ammonium pertechnate | tetragonal | technetium may be supplied in this form | [1] | ||
| AgTcO4 | silver pertechnate | tetragonal | [1] | |||
Use of pertechnetate to carry technetium-99m
A technetium-99m generator provides the pertechnetate containing the short-lived isotope 99mTc for medical uses. This compound is generated directly from molybdate held on alumina within the generator (see this topic for detail).
Uses in nuclear medicine
Pertechnetate has a wide variety of uses in diagnostic nuclear medicine. Since technetate(VII) can substitute for iodine in the Na/I symporter (NIS) channel in follicular cells of the thyroid gland, inhibiting uptake of iodine into the follicular cells, 99mTc-pertechnetate can be used as an alternative to 123I in imaging of the thyroid, although it specifically measures uptake and not organification.[2] It has also been used historically to evaluate for testicular torsion, although ultrasound is more commonly used in current practice, as it does not deliver a radiation dose to the testes. It is also used in labeling of autologus red blood cells for MUGA scans to evaluate left ventricular cardiac function, localization of gastrointestinal bleeding prior to embolization or surgical management, and in damaged red blood cells to detect ectopic splenic tissue.
It is actively accumulated and secreted by the mucoid cells of the gastric mucosa,[3] and therefore, technetate(VII) radiolabeled with Tc99m is injected into the body when looking for ectopic gastric tissue as is found in a Meckel's diverticulum with Meckel's scans.[4]
Uses of pertechnetate which do not rely on its radioactivity
All technetium salts are mildly radioactive, but some of them have explored use of the element for its chemical properties. In these uses, its radioactivity is incidental, and generally the least radioactive (longest-lived) isotopes of Tc are used. In particular, 99Tc (half-life 211,000 years) is used in corrosion research, because it is the decay product of the easily obtained commercial 99mTc isotope,[1] which is its nuclear isomer. In theory, the longest-lived technetium isotope, 98Tc (half-life 4.2 million years), would be the optimal isotope for this use.
Solutions of technetate(VII) react with the surface of iron to form technetium dioxide, in this way it is able to act as an anodic corrosion inhibitor.[5]
References
- Weaver, Jamie; Soderquist, Chuck Z.; Washton, Nancy M.; Lipton, Andrew S.; Gassman, Paul L.; Lukens, Wayne W.; Kruger, Albert A.; Wall, Nathalie A.; McCloy, John S. (21 February 2017). "Chemical Trends in Solid Alkali Pertechnetates". Inorganic Chemistry. 56 (5): 2533–2544. doi:10.1021/acs.inorgchem.6b02694. PMID 28221786.
- Ryo, U.Y.; Vaidya, P.V.; Schneider, A.B.; Bekerman, C; Pinsky, S.M. (1983). "Thyroid imaging agents: a comparison of I-123 and Tc-99m pertechnetate". Radiology. 148 (3): 819–822. doi:10.1148/radiology.148.3.6308711. PMID 6308711.
- Nuclear Imaging of Meckel's Diverticulum: A Pictorial Essay of Pitfalls Archived 2012-01-17 at the Wayback Machine S. Huynh, M.D., R. Amin, M.D., B. Barron, M.D., R. Dhekne, M.D., P. Nikolaidis, M.D., L. Lamki, M.D.. University of Texas Houston Medical School and Memorial Hermann - Texas Medical Center (TMC), St. Luke's Episcopal Hospital and Texas Children Hospital, Houston, Texas. Last Modified September 5, 2007
- Diamond, Robert; Rothstein, Robin; Alavi, Abass (1991). "The Role of Cimetidine-Enhanced Technetium 99m-Pertechnetate Imaging for Visualizing Meckel's Diverticulum" (PDF). The Journal of Nuclear Medicine. 32 (7): 1422–1424.
- Cartledge, G. H. (1973). "Twenty-Year Inhibition of Corrosion by the Pertechnetate Ion". Corrosion. 29 (9): 361–362. doi:10.5006/0010-9312-29.9.361.