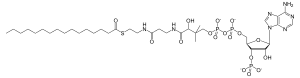
Palmitoyl-CoA
Palmitoyl-CoA is an acyl-CoA thioester. It is an "activated" form of palmitic acid and can be transported into the mitochondrial matrix by the carnitine shuttle system (which transports fatty acyl-CoA molecules into the mitochondria), and once inside can participate in β-oxidation. Alternatively, palmitoyl-CoA is used as a substrate in the biosynthesis of sphingosine (this biosynthetic pathway does not require transfer into the mitochonidria).[1][2]
 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.015.616 |
| KEGG | |
| MeSH | Palmitoyl+Coenzyme+A |
PubChem CID |
|
| |
| |
| Properties | |
| C37H66N7O17P3S | |
| Molar mass | 1004.94 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Biosynthesis
Palmitoyl CoA formed from palmitic acid, in the reaction below.[3]
This reaction is often referred to as the "activation" of a fatty acid. The activation is catalyzed by palmitoyl-coenzyme A synthetase and the reaction proceeds through a two step mechanism, in which palmitoyl-AMP is an intermediate.[4] The reaction is driven to completion by the exergonic hydrolysis of pyrophosphate[3].
The activation of fatty acids occurs in the cytosol and beta-oxidation occurs in the mitochondria. However, long chain fatty acyl-CoA cannot cross the mitochondrial membrane. If palmitoyl-CoA is to enter the mitchondria, it must react with carnitine in order to be transported across:
This transesterification reaction is catalyzed by carnitine palmitoyl transferase.[5] Palmitoyl-Carnitine may translocate across the membrane, and once on matrix side, the reaction proceeds in reverse as CoA-SH is recombined with palmitoyl-CoA, and released. Unattached carnitine is then shuttled back to the cytosolic side of mitochondrial membrane.
Beta-Oxidation
Once inside the mitochondrial matrix, palmitoyl-CoA may undergo β-oxidation. The full oxidation of palmitic acid (or palmitoyl-CoA) results in 8 acetyl-CoA's, 7 NADH, 7 H+
, and 7 FADH2.[6] The full reaction is below:
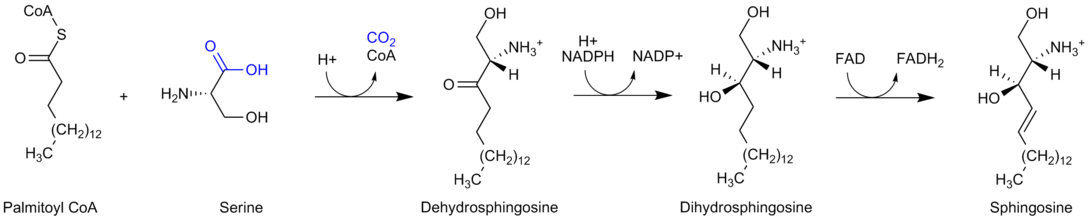
Sphingolipid Biosynthesis
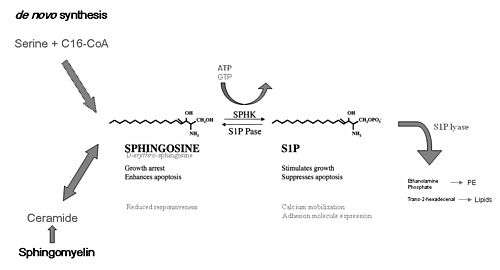
Palmitoyl-CoA is also the starting substrate, along with Serine, for sphingolipid biosynthesis. Palmitoyl CoA and Serine participate in a condensation reaction catalyzed by serine C-palmitoyltransferase (SPT), in which 3-ketosphinganine is formed. These reactions occur in the cytosol.[7]
Additional images
 Synthesis
Synthesis
References
- Brady, R.N.; DiMari, S.J.; Snell, E.E. (1969). "Biosynthesis of sphingolipid bases. 3. Isolation and characterization of ketonic intermediates in the synthesis of sphingosine and dihydrosphingosine by cell-free extracts of Hansenula ciferri". J. Biol. Chem. 244: 491–496. PMID 4388074.
- Stoffel, W.; Le Kim, D.; Sticht, G. (1968). "Biosynthesis of dihydrosphingosine in vitro". Hoppe-Seyler's Z. Physiol. Chem. 349: 664–670. doi:10.1515/bchm2.1968.349.1.664. PMID 4386961.
- Voet, Donald; Voet, Judith G.; Pratt, Charlotte W. (2016-02-29). Fundamentals of Biochemistry: Life at the Molecular Level. John Wiley & Sons. ISBN 978-1-118-91840-1.
- Bar–Tana, J.; Rose, G.; Brandes, R.; Shapiro, B. (1973-02-01). "Palmitoyl-coenzyme A synthetase. Mechanism of reaction". Biochemical Journal. 131 (2): 199–209. doi:10.1042/bj1310199. ISSN 0264-6021. PMC 1177459. PMID 4722436.
- Sharma, R. (2013), "Biochemical Mechanisms of Fatty Liver and Bioactive Foods", Bioactive Food as Dietary Interventions for Liver and Gastrointestinal Disease, Elsevier, pp. 709–741, doi:10.1016/b978-0-12-397154-8.00041-5, ISBN 978-0-12-397154-8
- Kamel, Kamel S.; Halperin, Mitchell L. (2017), "Ketoacidosis", Fluid, Electrolyte and Acid-Base Physiology, Elsevier, pp. 99–139, doi:10.1016/b978-0-323-35515-5.00005-1, ISBN 978-0-323-35515-5
- Michel, Christoph; van Echten-Deckert, Gerhild (1997-10-20). "Conversion of dihydroceramide to ceramide occurs at the cytosolic face of the endoplasmic reticulum". FEBS Letters. 416 (2): 153–155. doi:10.1016/s0014-5793(97)01187-3. ISSN 0014-5793. PMID 9369202.