Neoteny
Neoteny (/niˈɒtəni/)[1][2][3][4], also called juvenilization,[5] is the delaying or slowing of the physiological (or somatic) development of an organism, typically an animal. Neoteny is found in modern humans.[6] In progenesis (also called paedogenesis), sexual development is accelerated.[7]
Both neoteny and progenesis result in paedomorphism (or paedomorphosis), a type of heterochrony.[8] Some authors define paedomorphism as the retention of larval traits, as seen in salamanders.[9][10][11]
Both neoteny and progenesis cause the retention in adults of traits previously seen only in the young. Such retention is important in evolutionary biology, domestication and evolutionary developmental biology.
History and etymology

The origins of the concept of neoteny have been traced to the Bible (as argued by Ashley Montagu) and to the poet William Wordsworth's "The Child is the father of the Man" (as argued by Barry Bogin). The term itself was invented in 1885 by Julius Kollmann as he described the axolotl's maturation while remaining in a tadpole-like aquatic stage complete with gills, unlike other adult amphibians like frogs and toads.[12]
The word neoteny is borrowed from the German Neotenie, the latter constructed by Kollmann from the Greek νέος (neos, "young") and τείνειν (teínein, "to stretch, to extend"). The adjective is either "neotenic" or "neotenous".[13] For the opposite of "neotenic", different authorities use either "gerontomorphic"[14][15] or "peramorphic".[16] Bogin points out that Kollmann had intended the meaning to be "retaining youth", but had evidently confused the Greek teínein with the Latin tenere, which had the meaning he wanted, "to retain", so that the new word would mean "the retaining of youth (into adulthood)".[12]
In 1926 Louis Bolk described neoteny as the major process in humanization.[12] In his 1977 book Ontogeny and Phylogeny,[17] Stephen Jay Gould noted that Bolk's account constituted an attempted justification for "scientific" racism and sexism, but acknowledged that Bolk had been right in the core idea that humans differ from other primates in becoming sexually mature in an infantile stage of body development.[12]
In humans
Neoteny in humans is the slowing or delaying of body development, compared to non-human primates, resulting in features such as a large head, a flat face, and relatively short arms. These neotenic changes may have been brought about by sexual selection in human evolution. In turn, they may have permitted the development of human capacities such as emotional communication. However, humans also have relatively large noses and long legs, both peramorphic (not neotenic) traits. Some evolutionary theorists have proposed that neoteny was a key feature in human evolution.[18] Gould argued that the "evolutionary story" of humans is one where we have been "retaining to adulthood the originally juvenile features of our ancestors".[19] J. B. S. Haldane mirrors Gould's hypothesis by stating a "major evolutionary trend in human beings" is "greater prolongation of childhood and retardation of maturity."[5] Delbert D. Thiessen said that "neoteny becomes more apparent as early primates evolved into later forms" and that primates have been "evolving toward flat face."[20] However, in light of some groups using neoteny-based arguments to support racism, Gould also argued "that the whole enterprise of ranking groups by degree of neoteny is fundamentally unjustified" (Gould, 1996, pg. 150).[21] Doug Jones argued that human evolution's trend toward neoteny may have been caused by sexual selection in human evolution for neotenous facial traits in women by men with the resulting neoteny in male faces being a "by-product" of sexual selection for neotenous female faces.[22]
In domestic animals
Neoteny is seen in domesticated animals such as dogs and mice.[23] This is because there are more resources available, less competition for those resources, and with the lowered competition the animals expend less energy obtaining those resources. This allows them to mature and reproduce more quickly than their wild counterparts.[23] The environment that domesticated animals are raised in determines whether or not neoteny is present in those animals. Evolutionary neoteny can arise in a species when those conditions occur, and a species becomes sexually mature ahead of its "normal development". Another explanation for the neoteny in domesticated animals can be the selection for certain behavioral characteristics. Behavior is linked to genetics which therefore means that when a behavioral trait is selected for, a physical trait may also be selected for due to mechanisms like linkage disequilibrium. Often, juvenile behaviors are selected for in order to more easily domesticate a species; aggressiveness in certain species comes with adulthood when there is a need to compete for resources. If there is no need for competition, then there is no need for aggression. Selecting for juvenile behavioral characteristics can lead to neoteny in physical characteristics because, for example, with the reduced need for behaviors like aggression there is no need for developed traits that would help in that area. Traits that may become neotenized due to decreased aggression may be a shorter muzzle and smaller general size among the domesticated individuals. Some common neotenous physical traits in domesticated animals (mainly dogs, pigs, ferrets, cats, and even foxes) include: floppy ears, changes in reproductive cycle, curly tails, piebald coloration, fewer or shortened vertebra, large eyes, rounded forehead, large ears, and shortened muzzle.[24][25]
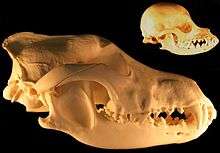
When the role of dogs expanded from just being working dogs to also being companions, humans started selective breeding dogs for morphological neoteny, and this selective breeding for "neoteny or paedomorphism" had the effect of enhancing the bond between humans and dogs.[26] Humans bred dogs to have more "juvenile physical traits" as adults such as short snouts and wide-set eyes which are associated with puppies, because people usually consider these traits to be more attractive. Some breeds of dogs with short snouts and broad heads such as the Komondor, Saint Bernard and Maremma Sheepdog are more morphologically neotenous than other breeds of dogs.[27] Cavalier King Charles spaniels are an example of selection for neoteny, because they exhibit large eyes, pendant-shaped ears and compact feet, giving them a morphology similar to puppies as adults.[26]
In 2004, a study that used 310 wolf skulls and over 700 dog skulls representing 100 breeds concluded that the evolution of dog skulls can generally not be described by heterochronic processes such as neoteny although some pedomorphic dog breeds have skulls that resemble the skulls of juvenile wolves.[28] By 2011, the finding by the same researcher was simply "Dogs are not paedomorphic wolves."[29]
In other species

Neoteny has been observed in many other species. It is important to note the difference between partial and full neoteny when looking at other species in order to distinguish between juvenile traits that are only advantageous in the short term and traits that provide a benefit throughout the organism's life; this might then provide some insight into the cause of neoteny in those species. Partial neoteny is the retention of the larval form beyond the usual age of maturation with the possibility of the development of sexual organs progenesis, but eventually the organism still matures into the adult form; this can be seen in Lithobates clamitans. Full neoteny is seen in Ambystoma mexicanum and some populations of Ambystoma tigrinum, which remain in their larval form for the duration of their life.[30][31] Lithobates clamitans is partially neotenous: it delays its maturation through the winter season, because it is not advantageous for it to metamorphose into the adult form until there are more resources available: it can find those resources much more easily in the larval form. This would fall under both of the main causes of neoteny; the energy required to survive in the winter as a newly formed adult is too costly, so the organism exhibits neotenous characteristics until a time when it is capable of better survival as an adult. Ambystoma tigrinum retains its neotenous features for a similar reason, however the retention is permanent due to the lack of resources available throughout its lifetime. This is another example of an environmental cause of neoteny in that the species retains juvenile characteristics because the environment limits the ability of the organism to fully come into its adult form. A few species of birds show partial neoteny. A couple of examples of such species are the manakin birds Chiroxiphia linearis and Chiroxiphia caudata. The males of both species retain their juvenile plumage into adulthood, but they eventually lose it once they are fully mature.[32] In certain species of birds the retention of juvenile plumage is often linked to the molting times within each species. In order to ensure there is no overlap between the molting and mating times, the birds may show partial neoteny in regards to their plumage so that the males do not attain their bright adult plumage before the females are prepared to mate. In this instance, neoteny is present because there is no need for the males to molt early and it would be a waste of energy for them to try to mate while the females are still immature.
Neoteny is commonly seen in flightless insects like the females in the order Strepsiptera. The flightless trait in insects has evolved many separate times; environments that may have contributed to the separate evolution of this trait are: high altitudes, isolation on islands, and insects that reside in colder climates.[33] These environmental factors may be responsible for the flightless trait, because in these situations it would be disadvantageous to have a population that is more dispersed, so flightlessness would be favored due to the boundaries it poses to dispersal. Also, in cooler temperatures heat is lost more rapidly through wings, thus the circumstance favors flightlessness. Another couple of main points to note about insects are that the females in certain groups become sexually mature without metamorphosing into adulthood, and some insects which grow up in certain conditions do not ever develop wings. Flightlessness in some female insects has been linked to higher fecundity, this would increase the fitness of the individual because the female is producing more offspring and therefore passing on more of her genes.[33] In those instances, neoteny occurs because it is more advantageous for the females to remain flightless in order to conserve energy which thereby increases their fecundity. Aphids are a great example of insects that may never develop wings due to their environmental setting. If resources are abundant there is no need to grow wings and disperse. When the nutrition of a host plant is abundant, aphids may not grow wings, remaining on the host plant for the duration of their lives; however, if the resources become diminished, their offspring may develop wings in order to disperse to other host plants.[34]
Two common environments that tend to favor neoteny are high-altitude and cool environments because neotenous individuals have a higher fitness than those that metamorphose into the adult form. This is because the energy required for metamorphosis is too costly for the individual's fitness, also the conditions favor neoteny due to the ability of neotenous individuals to utilize the available resources more easily.[35] This trend can be seen in the comparison of salamander species of lower and higher altitudes. The neotenous individuals have higher survivorship as well as higher fecundity than the salamanders that had gone to the adult form in the higher altitude and cooler environment.[35] Insects in cooler environments tend to show neoteny in flight because wings have a high surface area and lose heat quickly, thus it is not advantageous for insects in that environment to metamorphose into adults.[33]
Many species of salamander, and amphibians in general, are known to have neotenized characteristics because of the environment they live in. Axolotl and olm are species of salamander that retain their juvenile aquatic form throughout adulthood, examples of full neoteny. Gills are a common juvenile characteristic in amphibians that are kept after maturation; an example of this would be a comparison of the tiger salamander and the rough-skinned newt, both of which retain gills into adulthood.[30]
Pygmy chimpanzees (bonobos) share many physical characteristics with humans. A prime example is their neotenous skulls.[36] The shape of their skull does not change into adulthood; it only increases in size. This is due to sexual dimorphism and an evolutionary change in timing of development.[36] Juveniles became sexually mature before their bodies had fully developed into adulthood, and due to some selective advantage the neotenic structure of the skull remained in later generations.
In some species, energy costs result in neoteny, as in the insect families Gerridae, Delphacidae, and Carabidae. Many of the species in these families have smaller, neotenous wings or no wings at all.[34] Similarly, some cricket species shed their wings in adulthood,[37] while in beetles of the genus Ozopemon, the males (thought to be the first example of neoteny in the Coleoptera) are significantly smaller than the females, through inbreeding.[38] In the termite Kalotermes flavicollis, neoteny is seen in females during molting.[39]
In other species, environmental conditions cause neoteny, as in the northwestern salamander (Ambystoma gracile), where higher altitude is correlated with greater neotenic tendencies, perhaps to help conserve energy as mentioned above.[40] Similarly, neoteny is found in a few species of the crustacean family Ischnomesidae, which live in deep ocean waters.[41]
Subcellular neoteny
Neoteny is usually used to describe animal development; however, neoteny is also seen in the cell organelles. It was suggested that subcellular neoteny could explain why sperm cells have atypical centrioles. One of the two sperm centrioles of fruit fly exhibits the retention of “juvenile” centriole structure, which can be described as centriolar “neoteny”. This neotenic, atypical centriole, is known as the Proximal Centriole-Like. Typical centrioles form via a step by step process in which a cartwheel form, then develops to become a procentriole, and further matures into a centriole. The neotenic centriole of fruit fly resembles an early procentriole.[42]
See also
References
- "neoteny". Dictionary.com Unabridged. Random House. Retrieved April 21, 2019.
- "neoteny". The American Heritage Dictionary of the English Language (5th ed.). Boston: Houghton Mifflin Harcourt. Retrieved April 21, 2019.
- "neoteny". Oxford Dictionaries US Dictionary. Oxford University Press. Retrieved April 21, 2019.
- "neoteny". Merriam-Webster Dictionary. Retrieved April 21, 2019.
- Montagu, A. (1989). Growing Young. Bergin & Garvey: CT.
- Choi, Charles Q. (1 July 2009). "Being More Infantile May Have Led to Bigger Brains". Scientific American.
- Volkenstein, M. V. 1994. Physical Approaches to Biological Evolution. Springer-Verlag: Berlin, .
- Ridley, Mark (1985). Evolution. Blackwell.
- Whiteman, H.H. (1994). "Evolution of facultative paedomorphosis". Quarterly Review of Biology. 69 (2): 205–221. doi:10.1086/418540.
- Schell, S. C. Handbook of Trematodes of North America North of Mexico, 1985, pg. 22
- Ginetsinskaya, T.A. Trematodes, Their Life Cycles, Biology and Evolution. Leningrad, USSR: Nauka 1968. Translated in 1988, .
- Bogin, Barry (1999). Patterns of Human Growth. Cambridge University Press. pp. 157–169. ISBN 978-0-521-56438-0.
- Neoteny, The Free Dictionary. 2011. Accessed April 30, 2011.
- Henke, W. (2007). Handbook of paleoanthropology, Volume 1. Springer Books, NY.
- Hetherington, R. (2010). The Climate Connection: Climate Change and Modern Human Evolution. Cambridge University Press.
- Hall, B.K., Hallgrímsson, B. Monroe, W.S. (2008). Strickberger's evolution: the integration of genes, organisms and populations. Jones and Bartlett Publishers: Canada.
- Gould, Stephen Jay (1977). Ontogeny and Phylogeny. Cambridge, Massachusetts: Belknap (Harvard University Press). ISBN 978-0-674-63940-9.
- Shea, Brian T. (1989). "Heterochrony in human evolution: The case for neoteny reconsidered". American Journal of Physical Anthropology. 32 (S10): 69–101. doi:10.1002/ajpa.1330320505.
- Gould, S.J. A Biological Homage to Mickey Mouse.
- Thiessen, D.D. (1997). Bittersweet destiny: the stormy evolution of human behavior. Transaction Publishers, N.J.
- Gould, S.J. (1996). The mismeasure of man. W.W. Norton and Company, N.Y.
- Jones, D.; et al. (1995). "Sexual selection, physical attractiveness, and facial neoteny: Cross-cultural evidence and implications [and comments and reply]". Current Anthropology. 36 (5): 723–748. doi:10.1086/204427.
- Price, E. (1999). "Behavioral development in animals undergoing domestication". Applied Animal Behaviour Science. 65 (3): 245–271. doi:10.1016/S0168-1591(99)00087-8.
- Bertone, J. (2006). Equine geriatric medicine and surgery. Saunders, MI.
- Trut, L. N. (1999). "Early canid domestication: the farm-fox experiment". American Scientist. 87 (2): 160–169. doi:10.1511/1999.2.160.
- McGreevy, P.D. & Nicholas, F.W. (1999). Some Practical Solutions to Welfare Problems in Dog Breeding. In Animal Welfare. 8: 329-341.
- Beck, A.M. & Katcher, A.H. (1996). Between Pets and People: The Importance of Companionship. West Lafayette, Indiana: Purdue University Press. ISBN 1-55753-077-7
- Drake, Abby Grace, "Evolution and development of the skull morphology of canids: An investigation of morphological integration and heterochrony" (January 1, 2004). Doctoral Dissertations Available from Proquest. Paper AAI3136721. link
- Drake, Abby Grace (2011). "Dispelling dog dogma: An investigation of heterochrony in dogs using 3D geometric morphometric analysis of skull shape". Evolution & Development. 13 (2): 204–213. doi:10.1111/j.1525-142X.2011.00470.x. PMID 21410876.
- Swingle, W. (1922). "Experiments on the metamorphosis of neotenous amphibians". Journal of Experimental Zoology. 36 (4): 397–421. doi:10.1002/jez.1400360402.
- "Ambystoma tigrinum". Amphibia Web.
- Foster, M. (1987). "Delayed maturation, neoteny, and social system differences in two manakins of genus Chiroxyphia". Evolution. 41 (3): 547–558. doi:10.2307/2409256. JSTOR 2409256. PMID 28563802.
- Barbosa, P.; et al. (1989). "Life-history traits of forest-inhabiting flightless Lepidoptera". American Midland Naturalist. 122 (2): 262–274. doi:10.2307/2425912. JSTOR 2425912.
- Harrison, R (1980). "Dispersal polymorphisms in insects". Annual Review of Ecology and Systematics. 11: 95–118. doi:10.1146/annurev.es.11.110180.000523. JSTOR 2096904.
- Snyder, R (1956). "Comparative Features of the Life Histories of Ambystoma gracile (Baird) from Populations at Low and High Altitudes". Copeia. 1956 (1): 41–50. doi:10.2307/1439242. JSTOR 1439242.
- Shea, B. T. (1983). "Paedomorphosis and Neoteny in the Pygmy Chimpanzee". Science. 222 (4623): 521–522. doi:10.1126/science.6623093. JSTOR 1691380. PMID 6623093.
- Harrison, R (1980). "Dispersal Polymorphisms in Insects". Annual Review of Ecology and Systematics. 11: 95–118. doi:10.1146/annurev.es.11.110180.000523. JSTOR 2096904.
- Jordal, B. H.; Beaver, R. A.; Normark, B. B.; Farrell, B. D. (2002). "Extraordinary sex ratios and the evolution of male neoteny in sib-mating Ozopemon beetles". Biological Journal of the Linnean Society. 75 (3): 353–360. doi:10.1046/j.1095-8312.2002.00025.x.
- Soltani-Mazouni, N.; Bordereau, C. (1987). "Changes in the cuticle, ovaries and colleterial glands during the pseudergate and neotenic molt in Kalotermes flavicollis (FABR.) (Isoptera : Kalotermitidae)". International Journal of Insect Morphology and Embryology. 16 (3–4): 221–225. doi:10.1016/0020-7322(87)90022-5.
- Eagleson, G.; McKeown, B. (1978). "Changes in thyroid activity of Ambystoma gracile (Baird) during different larval, transforming, and postmetamorphic phases". Canadian Journal of Zoology. 56 (6): 1377–1381. doi:10.1139/z78-190.
- Brokeland, W.; Brandt, A. (2004). "Two new species of Ischnomesidae (Crustacea: Isopoda) from the Southern Ocean displaying neoteny". Deep-Sea Research Part II. 51 (14–16): 1769–1785. doi:10.1016/j.dsr2.2004.06.034.
- In The Golgi Apparatus and Centriole: Functions, Interactions and Role in Disease. M. Kloc, editor. Springer International Publishing, Cham. 3-15, https://link.springer.com/chapter/10.1007%2F978-3-030-23173-6_1
Further reading
- Wehr, Paul; MacDonald, K; Linder, R; Yeung, G (2001). "Stabilizing and directional selection on facial paedomorphosis". Human Nature. 12 (4): 383–402. doi:10.1007/s12110-001-1004-z. PMID 26192413.
- Bergstorm, Carl T. & Dugatkin, Lee Alan (2012). Evolution, W.W. Norton