Non-contact thermography
Non-contact thermography, thermographic imaging, or medical thermology is the field of thermography that uses infrared images of the human skin to assist in the diagnosis and treatment of medical conditions. Medical thermology is sometimes referred to as medical infrared imaging or tele-thermology and utilizes thermographic cameras. According to the American Academy of Thermology, Medical Thermology practitioners are licensed health care practitioners who utilize IR imaging in consistent with medically established paradigms of care. Non-medically licensed alternative practitioners who are not held to the same standard may offer thermography services but that should not be confused with the field of medical thermology.
| Thermography (medical) | |
|---|---|
| ICD-9 | 88.8 |
| MeSH | D013817 |
Restated, medical thermology is the use of infrared (IR) imaging to assess skin temperature as an extension of the clinician's physical exam to aid in the formation of a medical diagnosis or treatment plan. Medical Thermology does not condone those who purport that "Thermography" can find disease by looking for areas of the body that have abnormal heat or irregular blood flow. IR imaging simply does not does not have the ability to assess temperature beyond the surface of the skin.
Thermography is a physiologic study and is not a replacement for structural studies such as X-Ray, MRI, or Mammography. As a physiologic study however, medical thermology has many health related indications. The American Academy of Thermology (AAT) (www.aathermology.org)[1] has published internationally peer reviewed guidelines for neuro-musculoskeletal (MSK), breast, veterinary, and oral-systemic disease. [2]
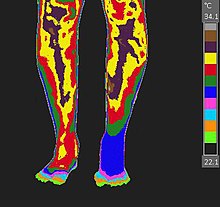
Examples of neuro-musculoskeletal indications for medical thermology include Reflex Sympathetic Dystrophy (RSD), Chronic Regional Pain Syndrome (CRPS), Dystautonomia, Migraine, Fibromyalgia (and other weather sensitive pain syndromes), thoracic outlet syndrome, and vaso- motor migraine/head aches such as Barre-Lieou syndrome. This is especially true when used to monitor the results of a cold stress (cold presser) test.[3]
While there are several clinical studies showing the effectiveness of thermography in detecting temperature abnormalities of breast skin, breast thermography is only approved as an adjunct modality only for breast disease and/or cancer.[4] In that regard it is a breast risk health assessment only. A summary of some of the clinical trials for breast thermography is available in this white paper from UE LifeSciences pdf.
Veterinary thermography indications include, but are not limited to, assessment of shoring, limb inflammation, and sweating disorders.[5]
When properly used, a commonly known oral-systemic indication for IR imaging is temperature measurement for fever screening. While there are several other oral-systemic indications out lined in the American Academy of Thermology's Oral -Systemic Guidelines. Whole body studies are not performed for medical purposes.[6]
Telethermography systems are regulated as a medical device under 21 CFR 884.2980.
Medical Thermology versus Thermography
There is a difference between Medical Thermology as promulgated by medically based organizations such as the American Academy of Thermology (AAT), and thermography as practiced by alternative providers or physicians who overstate the benefits of thermography. As a result of this disparity organizations such as the FDA, ISO, and the AAT have published Guidelines and best use practices to help educate medical providers and the public to recognize the difference between providers who provide medical thermology services and those that offer something else.[7]

Due to misunderstandings of the technology, the complex physiologic nature that thermographic imaging provides insight into, and large disparities between low end and medical grade thermal imaging cameras there is a lot of misinformation that tarnishes the industry. The Coronavirus pandemic is an excellent example to illustrate the point. Almost anyone can buy an inexpensive infrared measuring device intended for industrial use that has been re-marketed for fever screening, but said devices are not FDA approved for the same, may lack the specificity or sensitivity to be used for that indication, and the end users receive no training in its proper application. In an effort to address this problem the AAT has published both free and paid versions of IR Temperature Measurement Fever Screening training courses. The Level I course is intended for individuals who have financial restraint and have to use non-medical grade cameras. The course provides protocols to help overcome the shortcomings of these devices. Level II training is directed toward businesses and agencies that are can afford a medical grade system and have the resources to offer screening that is fully compliant with FDA, ISO, and AAT recommended best use practices.[8]
Thermography has been promoted by some alternative medicine practitioners as a means to diagnose cancer, although it is not effective for this purpose. Health Canada has issued "cease and desist" orders to clinics offering breast thermography as a cancer diagnostic device because thermography cameras are not licensed as a medical device in Canada, and because thermography for cancer detection is viewed as ineffective by medical experts.[9] The FDA has issued a public warning notice stating that breast thermography is not an alternative to mammography[10] and has ordered Joseph Mercola to stop making excessive claims for thermography.[11]
Thermography is discouraged in North America by the American Cancer Society, radiologists and the FDA for early breast cancer detection. Advertisements in the United Kingdom have been found to be misleading.[12]
The FDA has cleared thermography only as an adjunct method of screening. "Thermography devices have been cleared by the FDA for use as an adjunct, or additional, tool for detecting breast cancer." However, they stop short of recommending it, citing the lack of evidence of its effectiveness.[13] The AAT has published several Position Papers, including statements on Breast Thermography that clearly delineate its utility as an adjudicative breast risk health assessment only.[14]
See also
References
- "American Academy of Thermology | American Academy of Thermology". Retrieved 2020-06-19.
- "Guidelines | American Academy of Thermology". Retrieved 2020-06-19.
- "Guidelines for Neuro-Musculoskeletal Infrared Medical Thermography And Sympathetic Skin Response (SSR) Studies | American Academy of Thermology". Retrieved 2020-06-19.
- Commissioner, Office of the. "Press Announcements - FDA issues warning letter to clinic illegally marketing unapproved thermography device, warns consumers to avoid using thermography devices to detect breast cancer". www.fda.gov. Retrieved 2 March 2019.
- "Veterinary Guidelines for Infrared Thermography | American Academy of Thermology". Retrieved 2020-06-19.
- "Guidelines for Oral Systemic Thermography | American Academy of Thermology". Retrieved 2020-06-19.
- "Thermology | American Academy of Thermology". Retrieved 2020-06-19.
- "Fever Screening Courses | AAT Online Training Center". courses.aathermology.org. Retrieved 2020-06-19.
- Clinics ordered to stop 'useless' breast cancer tests, CBC News, Nov 27 2012
- FDA Safety Communication: Breast Cancer Screening - Thermography is Not an Alternative to Mammography, the U.S. Food and Drug Administration June 2, 2011
- Tsouderos, Trine (25 April 2012). "FDA warns doctor: Stop touting camera as disease screening tool". Chicago Tribune. Retrieved 8 January 2013.
- "ASA Adjudication on Medical Thermal Imaging Ltd". Advertising Standards Authority. 2013-01-09. Retrieved January 5, 2015.
- Commissioner, Office of the. "Consumer Updates - Thermogram No Substitute for Mammogram".
- "Position Papers | American Academy of Thermology". Retrieved 2020-06-19.