Nitric-oxide reductase
Nitric oxide reductase, an enzyme, catalyzes the reduction of nitric oxide (NO) to nitrous oxide (N2O).[1][2][3][4] The enzyme participates in nitrogen metabolism and in the microbial defense against nitric oxide toxicity. The catalyzed reaction may be dependent on different participating small molecules: Cytochrome c (EC: 1.7.2.5, Nitric oxide reductase (cytochrome c)), NADPH (EC:1.7.1.14), or Menaquinone (EC:1.7.5.2).
| nitric oxide reductase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC number | 1.7.2.5 | ||||||||
| CAS number | 37256-43-2 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
Nomenclature
Nitric oxide reductase was assigned Enzyme Commission number (EC) 1.7.2.5. Enzyme Commission numbers are the standard naming system used for enzymes.[5] The EC identifies the class, subclass, sub-subclass, and serial number of the enzyme.[5] Nitric oxide reductase is in Class 1, therefore it is an oxidoreductases.[5]
Nitric oxide reductase belongs to the family of oxidoreductases, specifically those acting on other nitrogenous compounds as donors with other acceptors. The systematic name of this enzyme class is nitrous-oxide:acceptor oxidoreductase (NO-forming). Other names in common use include nitrogen oxide reductase, and nitrous-oxide:(acceptor) oxidoreductase (NO-forming).
Function
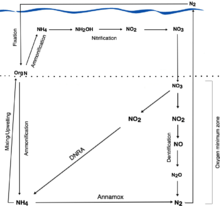
Organisms reduce nitrate (NO3−) to nitrogen gas (N2) through the process of denitrification, see Figure 1.[1][2] Two important intermediates of the reduction pathway are nitric oxide (NO) and nitrous oxide (N2O).[1][2] The reducing reaction that transforms NO into N2O is catalyzed by nitric oxide reductase (NOR).[1][2][3][4]
NO is reduced to N2O also to prevent cellular toxicity.[4][6] N2O, a potent greenhouse gas, is released.[1][4]
Reaction
In enzymology, a nitric oxide reductase (NOR) catalyzes the chemical reaction:
- 2 NO + 2 e− + 2 H+ N2O + H2O[4]
The enzyme acts on 2 nitric oxide (substrate).[2] The enzyme converts NO, electrons and protons to products: nitrous oxide, and H2O.[2]
Inputs: 2 molecules of NO, 2 electrons, 2 protons[2]
Outputs: 1 molecule of N2O, 1 molecule of H2O[2]
Mechanism
NOR catalyzes the formation of nitrogen to nitrogen (N--N) bonding.[1][3][6] The conformation changes of the active site and attached ligands (ie. Glu211) allows NO to be positioned in the crowded binuclear center and form N--N bonds.[4]
The precise mechanism of catalysis is still unknown, although hypotheses have been proposed.[3][4]
Cordas et al. 2013 proposes three options: the trans-mechanism, the cis-FeB and the cis-heme b3 mechanisms.[3]
Based on the structure of the enzyme, Shiro 2012 proposes the following mechanism: (1) NO molecules bind at the binuclear center, (2) electrons are transferred from the ferrous irons to the NO, (3) charged NO molecules have the potential to form N to N bonds, and (4) N to O bonds are potentially broken by water, allowing for the N2O and H2O to be released.[4]
According to Hino et al. 2010, the changing charge of the active site causes NO to bind, form N2O and leave the enzyme. The NOR active site is positioned near two hydrogen bound glutamic acids (Glu). The Glu groups provide an electron-negative charge to the active site.[1] The electro-negative charge reduces the reaction potential for heme b3 and allows NO to bind to the binuclear activation site.[1] Glu residues also provide protons needed for removal of N2O and production of H2O.[1]
Structure
Subunits
NOR is made up of two subunits, NorC (small) and NorB (large), with a binuclear iron centre.[1][3][4] The binuclear iron center is the active site.[1][2][3][4] It is composed of two b-type hemes and a non-heme iron (FeB).[1][2][3][4] The ligands are connected through a μ-oxo bridge.[3] Histidine (His) residues are attached to the heme b3 in the small subunit.[1] The hydrophilic region of the larger subunit has His and methionine (Met) ligands.[1] Structure is similar to cytochrome oxidases.[1][4]
The active site is conserved between cNOR and qNOR, although differences (ie. heme type) occur between cNOR and qNOR.[4]
Species distribution
Bacteria, archaea and fungi use NOR.[4][6] qNOR is found in denitrifying bacteria and archaea, as well as pathogenic bacteria not involved in denitrification.[4] Denitrifying fungi reduce NO using P-450nor soluble enzyme.[6]
Types
Three types of NOR were identified from bacteria: cNOR, qNOR, and qCuNOR.[3] cNOR was found in denitrifying bacteria: Paracoccus denitrificans, Halomonas halodenitrificans, Pseudomonas nautica, Pseudomonas stutzeri, and Pseudomonas aeruginosa.[3] cNOR was first isolated from P. aeruginosa.[1][4] qNOR was isolated from Geobacillus stearothermophilus.[4]
References
- Hino T, Matsumoto Y, Nagano S, Sugimoto H, Fukumori Y, Murata T, et al. (December 2010). "Structural basis of biological N2O generation by bacterial nitric oxide reductase". Science. 330 (6011): 1666–70. Bibcode:2010Sci...330.1666H. doi:10.1126/science.1195591. PMID 21109633.
- Collman JP, Yang Y, Dey A, Decréau RA, Ghosh S, Ohta T, Solomon EI (October 2008). "A functional nitric oxide reductase model". Proceedings of the National Academy of Sciences of the United States of America. 105 (41): 15660–5. Bibcode:2008PNAS..10515660C. doi:10.1073/pnas.0808606105. PMC 2572950. PMID 18838684.
- Cordas CM, Duarte AG, Moura JJ, Moura I (March 2013). "Electrochemical behaviour of bacterial nitric oxide reductase-evidence of low redox potential non-heme Fe(B) gives new perspectives on the catalytic mechanism". Biochimica et Biophysica Acta (BBA) - Bioenergetics. 1827 (3): 233–8. doi:10.1016/j.bbabio.2012.10.018. PMID 23142527.
- Shiro Y (October 2012). "Structure and function of bacterial nitric oxide reductases: nitric oxide reductase, anaerobic enzymes". Biochimica et Biophysica Acta. 1817 (10): 1907–13. doi:10.1016/j.bbabio.2012.03.001. PMID 22425814.
- Moss GP, Webb EC, et al. (International Union of Biochemistry and Molecular Biology. Nomenclature Committee.; IUPAC-IUBMB Joint Commission on Biochemical Nomenclature) (1992). Enzyme nomenclature. Academic Press. ISBN 0122271645. OCLC 715593348.
- Hendriks J, Oubrie A, Castresana J, Urbani A, Gemeinhardt S, Saraste M (August 2000). "Nitric oxide reductases in bacteria". Biochimica et Biophysica Acta (BBA) - Bioenergetics. 1459 (2–3): 266–73. doi:10.1016/S0005-2728(00)00161-4. PMID 11004439.
- Yeung N, Lin YW, Gao YG, Zhao X, Russell BS, Lei L, et al. (December 2009). "Rational design of a structural and functional nitric oxide reductase". Nature. 462 (7276): 1079–82. Bibcode:2009Natur.462.1079Y. doi:10.1038/nature08620. PMC 4297211. PMID 19940850.
Further reading
- Heiss B, Frunzke K, Zumft WG (June 1989). "Formation of the N-N bond from nitric oxide by a membrane-bound cytochrome bc complex of nitrate-respiring (denitrifying) Pseudomonas stutzeri". Journal of Bacteriology. 171 (6): 3288–97. doi:10.1128/jb.171.6.3288-3297.1989. PMC 210048. PMID 2542222.
- Gardner AM, Helmick RA, Gardner PR (March 2002). "Flavorubredoxin, an inducible catalyst for nitric oxide reduction and detoxification in Escherichia coli". The Journal of Biological Chemistry. 277 (10): 8172–7. doi:10.1074/jbc.M110471200. PMID 11751865.