Muniscins
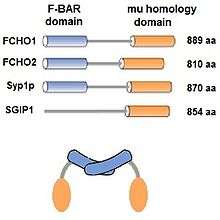
The muniscin protein family was initially defined in 2009[4] as proteins having 2 homologous domains that are involved in clathrin mediated endocytosis (CME) and have been reviewed.[5] In addition to FCHO1, FCHO2 and Syp1,[6][7] SGIP1 is also included in the family because it contains the μ (mu) homology domain and is involved in CME, even though it does not contain the F-BAR domain[1][8]

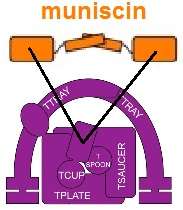
Muniscins are known as alternate cargo adaptors. That is, they participate in selecting which cargo molecules are internalized via CME.[5] Additionally, the structure of the dimer, with its concave face oriented toward the plasma membrane, is thought to help curve the membrane as the clathrin coated pit forms.[5] The muniscins are early arriving proteins involved in CME.[5] FCHO proteins are required for CME,[9] but do not appear to be required to initiate CME.[10]
The μ homology domain of muniscins has been reported to have evolved from part of an ancient cargo adaptor protein complex named TSET.[11]
References
- Umasankar PK, Ma L, Thieman JR, Jha A, Doray B, Watkins SC, Traub LM (2014). "A clathrin coat assembly role for the muniscin protein central linker revealed by TALEN-mediated gene editing". eLife. 3. doi:10.7554/eLife.04137. PMC 4215538. PMID 25303365.
- Shimada A, Yamaguchi A, Kohda D (2016). "Structural basis for the recognition of two consecutive mutually interacting DPF motifs by the SGIP1 μ homology domain". Scientific Reports. 6: 19565. doi:10.1038/srep19565. PMC 4731787. PMID 26822536.
- Weissenhorn W (2005). "Crystal structure of the endophilin-A1 BAR domain". Journal of Molecular Biology. 351 (3): 653–61. doi:10.1016/j.jmb.2005.06.013. PMID 16023669.
- Reider A, Barker SL, Mishra SK, Im YJ, Maldonado-Báez L, Hurley JH, Traub LM, Wendland B (2009). "Syp1 is a conserved endocytic adaptor that contains domains involved in cargo selection and membrane tubulation". The EMBO Journal. 28 (20): 3103–16. doi:10.1038/emboj.2009.248. PMC 2771086. PMID 19713939.
- Robinson MS (2015). "Forty Years of Clathrin-coated Vesicles". Traffic. 16 (12): 1210–38. doi:10.1111/tra.12335. PMID 26403691.
- anon. "SYP1 [Saccharomyces cerevisiae]". ncbi.nlm.nih.gov/. ncbi.nlm.nih.gov/. Retrieved 26 May 2017.
- Boettner DR, D'Agostino JL, Torres OT, Daugherty-Clarke K, Uygur A, Reider A, Wendland B, Lemmon SK, Goode BL (2009). "The F-BAR protein Syp1 negatively regulates WASp-Arp2/3 complex activity during endocytic patch formation". Current Biology. 19 (23): 1979–87. doi:10.1016/j.cub.2009.10.062. PMC 2828323. PMID 19962315.
- Dergai O, Novokhatska O, Dergai M, Skrypkina I, Tsyba L, Moreau J, Rynditch A (2010). "Intersectin 1 forms complexes with SGIP1 and Reps1 in clathrin-coated pits". Biochemical and Biophysical Research Communications. 402 (2): 408–13. doi:10.1016/j.bbrc.2010.10.045. PMID 20946875.
- Henne WM, Boucrot E, Meinecke M, Evergren E, Vallis Y, Mittal R, McMahon HT (2010). "FCHo proteins are nucleators of clathrin-mediated endocytosis". Science. 328 (5983): 1281–4. doi:10.1126/science.1188462. PMC 2883440. PMID 20448150.
- Cocucci E, Aguet F, Boulant S, Kirchhausen T (2012). "The first five seconds in the life of a clathrin-coated pit". Cell. 150 (3): 495–507. doi:10.1016/j.cell.2012.05.047. PMC 3413093. PMID 22863004.
- Hirst J, Schlacht A, Norcott JP, Traynor D, Bloomfield G, Antrobus R, Kay RR, Dacks JB, Robinson MS (2014). "Characterization of TSET, an ancient and widespread membrane trafficking complex". eLife. 3: e02866. doi:10.7554/eLife.02866. PMC 4031984. PMID 24867644.