Methylcyclohexane
Methylcyclohexane is an organic compound with the molecular formula is CH3C6H11. Classified as saturated hydrocarbon, it is a colourless liquid with a faint odor. Methylcyclohexane is used as a solvent. It is mainly converted in naphtha reformers to toluene.[4] Methylcyclohexane is also used in some correction fluids (such as White-Out) as a solvent.
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
methylcyclohexane | |||
| Other names
Hexahydrotoluene Cyclohexylmethane Toluene hexahydride | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChemSpider | |||
| ECHA InfoCard | 100.003.296 | ||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C7H14 | |||
| Molar mass | 98.189 g·mol−1 | ||
| Appearance | Colourless liquid | ||
| Odor | faint, benzene-like[1] | ||
| Density | 0.77 g/cm3 | ||
| Melting point | −126.3 °C (−195.3 °F; 146.8 K) | ||
| Boiling point | 101 °C (214 °F; 374 K) | ||
| 0.014 g/l at 25 °C[2] | |||
| Vapor pressure | 37 mmHg (20°C)[1] 49.3 hPa at 20.0 °C | ||
| -78.91·10−6 cm3/mol | |||
| Hazards | |||
| Main hazards | severe fire hazard | ||
| Safety data sheet | [2] | ||
| GHS pictograms |     | ||
| GHS Signal word | Danger | ||
GHS hazard statements |
H225, H304, H315, H336, H410[2] | ||
| P210, P235, P301+310, P331, P370+378, P403[2] | |||
| NFPA 704 (fire diamond) | |||
| Flash point | −4 °C (25 °F; 269 K)[2] Closed cup | ||
| 283 °C (541 °F; 556 K)[2] | |||
| Explosive limits | 1.2%-6.7%[1][2] | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose) |
2250 mg/kg (mouse, oral)[3] | ||
LC50 (median concentration) |
10172 ppm (mouse, 2 hr) 10,000-12,500 ppm (mouse, 2 hr) 15227 ppm (rabbit, 1 hr)[3] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible) |
TWA 500 ppm (2000 mg/m3)[1] | ||
REL (Recommended) |
TWA 400 ppm (1600 mg/m3)[1] | ||
IDLH (Immediate danger) |
1200 ppm[1] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Production and use
It can be also produced by hydrogenation of toluene:
- CH3C6H5 + 3 H2 → CH3C6H11
Methylcyclohexane, as a component of a mixture, is usually dehydrogenated to toluene, which increases the octane rating of gasoline.[4]
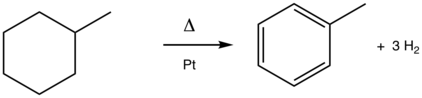 The conversion of methylcyclohexane to toluene is a classic aromatization reaction. This platinum (Pt)-catalyzed process is practiced on scale in the production of gasoline from petroleum.[5]
The conversion of methylcyclohexane to toluene is a classic aromatization reaction. This platinum (Pt)-catalyzed process is practiced on scale in the production of gasoline from petroleum.[5]
It is also one of a host substances in jet fuel surrogate blends, e.g., for Jet A fuel.[6][7]
Structure
Methylcyclohexane is a monosubstituted cyclohexane because it has one branching via the attachment of one methyl group on one carbon of the cyclohexane ring. Like all cyclohexanes, it can interconvert rapidly between two chair conformers. The lowest energy form of this monosubstituted methylcyclohexane occurs when the methyl group occupies an equatorial rather than an axial position. This equilibrium is embodied in the concept of A value. In the axial position, the methyl group experiences steric crowding (steric strain) because of the presence of axial hydrogen atoms on the same side of the ring (known as the 1,3-diaxial interactions). There are two such interactions, with each pairwise methyl/hydrogen combination contributing approximately 7.61 kJ/mol of strain energy. The equatorial conformation experiences no such interaction, and so it is the energetically favored conformation.
Flammability and toxicity
Methylcyclohexane is flammable.
Furthermore, it is considered "very toxic to aquatic life".[9] Note, while methylcyclohexane is a substructure of 4-methylcyclohexanemethanol (MCHM), it is distinct in its physical, chemical, and biological (ecologic, metabolic, and toxicologic) properties.[10]
References
- NIOSH Pocket Guide to Chemical Hazards. "#0406". National Institute for Occupational Safety and Health (NIOSH).
- Sigma-Aldrich Co., Methylcyclohexane. Retrieved on 2017-11-21.
- "Methylcyclohexane". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- M. Larry Campbell. "Cyclohexane" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2012. doi:10.1002/14356007.a08_209.pub2
- Gary, J.H.; Handwerk, G.E. (1984). Petroleum Refining Technology and Economics (2nd ed.). Marcel Dekker, Inc. ISBN 0-8247-7150-8.
- Tim Edwards, Meredith Colket, Nick Cernansky, Fred Dryer, Fokion Egolfopoulos, Dan Friend, Ed Law, Dave Lenhert, Peter Lindstedt, Heinz Pitsch, Adel Sarofim, Kal Seshadri, Mitch Smooke, Wing Tsang & Skip Williams, 2007, AIAA2007-770: Development of an Experimental Database and Kinetic Models for Surrogate Jet Fuels, 45th AIAA Aerospace Sciences Meeting and Exhibit, 8-11 January 2007, Reno, Nevada, DOI 10.2514/6.2007-770, see , accessed 27 May 2014.
- Meredith Colket, Tim Edwards, Fred Dryer, Skip Williams, Nicholas Cernansky, David Miller, Fokion Egolfopoulos, Frederick Dryer & Josette Bellan, 2008, AIAA 2008-972: Identification of Target Validation Data for Development of Surrogate Jet Fuels, 46th AIAA Aerospace Sciences Meeting and Exhibit, 8-11 January 2007, Reno, Nevada, DOI 10.2514/6.2008-972, see , accessed 27 May 2014.
- D. Bryce-Smith and E. T. Blues "Unsolvated n-Butylmagnesium Chloride" Org. Synth. 1967, 47, 113. doi:10.15227/orgsyn.047.0113
- Chevron Phillips, 2014, "Material Safety Data Sheet: Methylcyclohexane (v. 1.5)", see , accessed 23 May 2014.
- CDC, 2014, "Methylcyclohexane," NIOSH Pocket Guide to Chemical Hazards, see , accessed 27 May 2014.