Magnesite
Magnesite is a mineral with the chemical formula MgCO
3 (magnesium carbonate). Iron, manganese, cobalt and nickel may occur as admixtures, but only in small amounts.
| Magnesite | |
|---|---|
 Magnesite crystals from Brazil (11.4 x 9.2 x 3.6 cm) | |
| General | |
| Category | Carbonate mineral |
| Formula (repeating unit) | MgCO3 |
| Strunz classification | 5.AB.05 |
| Crystal system | Trigonal |
| Crystal class | Hexagonal scalenohedral (3m) H-M symbol: (3 2/m) |
| Space group | R3c |
| Identification | |
| Color | Colorless, white, pale yellow, pale brown, faintly pink, lilac-rose |
| Crystal habit | Usually massive, rarely as rhombohedrons or hexagonal prisms |
| Cleavage | [1011] perfect |
| Fracture | Conchoidal |
| Tenacity | Brittle |
| Mohs scale hardness | 3.5 – 4.5 |
| Luster | Vitreous |
| Streak | white |
| Diaphaneity | Transparent to translucent |
| Specific gravity | 3.0 – 3.2 |
| Optical properties | Uniaxial (-) |
| Refractive index | nω=1.508 – 1.510 nε=1.700 |
| Birefringence | 0.191 |
| Fusibility | infusible |
| Solubility | Effervesces in hot HCl |
| Other characteristics | May exhibit pale green to pale blue fluorescence and phosphorescence under UV; triboluminescent |
| References | [1][2][3][4] |
Occurrence
Magnesite occurs as veins in and an alteration product of ultramafic rocks, serpentinite and other magnesium rich rock types in both contact and regional metamorphic terrains. These magnesites are often cryptocrystalline and contain silica in the form of opal or chert.
Magnesite is also present within the regolith above ultramafic rocks as a secondary carbonate within soil and subsoil, where it is deposited as a consequence of dissolution of magnesium-bearing minerals by carbon dioxide in groundwaters.
Isotopic Structure: Clumped Isotope
The recent advancement in the field of stable isotope geochemistry is the study of isotopic structure of minerals and molecules. This requires study of molecules with high resolutions looking at bonding scenario (how heavy isotopes are bonded to each other)- leading to knowledge of stability of molecule depending on its isotopic structure.
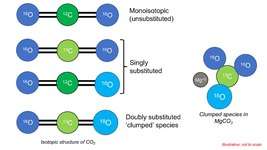
Oxygen has three stable isotopes (16O, 17O and 18O) and Carbon has two (13C, 12C). A 12C16O2 molecule (composed only with most abundant isotopes of constituent elements) is called a 'monoisotopic' species. When only one atom is replaced with heavy isotope of any constituent element (ie, 13C16O2), it is called a 'singly-substituted' species. Likewise, when two atoms are simultaneously replaced with heavier isotopes (eg., 13C16O18O), it is called a 'doubly substituted' species. The 'clumped' species (13C16O18O) for CO2 is a doubly substituted CO2 molecule. Isotopically substituted molecules have higher mass. As a consequence, molecular vibration reduces and the molecule develops a lower zero point energy (see Kinetic isotope effect).
The abundances of certain bonds in certain molecules are sensitive to temperature at which it formed (e.g., abundance of 13C16O18O in carbonates[5] as 13C-18O bond). This information has been exploited to form the foundation of clumped isotope geochemistry. Clumped isotope thermometers have been established for carbonate minerals like dolomite,[6][7] calcite,[8] siderite[9] etc and non-carbonate compounds like methane[10] and oxygen.[11] Depending on the strength of cation-carbonate oxygen (ie, Mg-O, Ca-O) bonds- different carbonate minerals can form or preserve clumped isotopic signatures differently.
Measurements and Reporting
Clumped isotopic analysis has certain aspects to it. These are:
Digestion, Analysis and Acid Fractionation Correction
Clumped isotopic analysis is usually done by gas source mass spectrometry where the CO2 liberated from magnesite by phosphoric acid digestion is fed into the isotope ratio mass spectrometer. In such scenario, one needs to ensure that liberation of CO2 from magnesite is complete. Digesting magnesite is hard since it takes a long time and different labs report different digestion times and temperatures (from 12 hours at 100oC[12] to 1 hour at 90oC[13] in phosphoric acid). Due to digestion at this high temperature, some of the 13C-18O bonds in the liberated CO2 are broken (leading to reduction in abundance of 'clumped' CO2) during phosphoric acid digestion of carbonates. To account for this additional (analytical artifact), a correction called the 'acid fractionation correction' is added to the magnesite clumped isotope value obtained at temperature of digestion.
Since the CO2 gas is liberated from carbonate mineral during acid digestion, leaving one O behind- a fractionation occurs, and the isotopic composition of the analyzed CO2 gas needs to be corrected for this. For magnesite, the most reliable fractionation factor(α) equation is given as:[14]
103ln(α) = [(6.845 ± 0.475)∗105/T2] + (4.22 ± 0.08); T in K
Different researchers have also used other fractionation factors like dolomite fractionation factor.[15]
Standards
While measuring samples of unknown composition, it is required to measure some standard materials (see Reference materials for stable isotope analysis). With internal standards and reference materials, analytical session is routinely monitored. Standard materials are majorly calcite and marble.
Δ47 - Temperature Calibration
To convert clumped isotope data into temperature, a calibration curve is required which expresses the functional form of temperature dependence of clumped isotope composition. No mineral specific calibration exists for magnesite. Based on some experimental data[13] where mineral precipitation temperature and clumped isotope derived temperature doesn't match, a need of mineral specific calibration emerges. The mismatch arises since bonding in magnesite is different from calcite/dolomite and/or acid digestion is conducted at higher temperature.
Magnesite-Water and CO2-Magnesite Isotope Fractionation Factors
Using clumped isotope derived temperature, C and O isotopic composition of the parental fluid can be calculated using known magnesite-fluid isotope fractionation factors, since fractionation is temperature dependent. Reported magnesite-fluid O and C isotope fractionation factors in literature are not in agreement with each other.[13] The fractionation behaviors have not been substantiated by experimental observation.
Factors Controlling Isotopic Structure in Magnesite
Conversion from Hydrous Mg-Carbonates to Magnesite
In low temperature, thus, hydrous Mg-carbonates (hydromagnesite, nesquehonite etc.) form. It is possible to convert these phases into magnesite by changing temperature by mineral dissolution-precipitation or dehydration. While so happens, an isotope effect associated can control the isotopic composition of precipitated magnesite.
Disequilibrium
Disequilibrium processes like degassing, rapid CO2 uptake etc. modify clumped isotopic composition of carbonate minerals specifically at low temperatures. They variably enrich or deplete the system in heavy isotopes of C and O. Since clumped isotope abundance depends on abundance of isotopes of C and O, they are also modified. Another very prominent effect here is that of pH of precipitating fluid.[16] As pH of precipitating fluid changes, DIC pool is affected and isotopic composition of precipitating carbonate changes.

Mineral Structure and Later Thermal Effects
Crystalline and cryptocrystalline magnesites have very different mineral structures. While crystalline magnesite has a well developed crystal structure, the cryptocrystalline magnesite is amorphous- mostly aggregate of fine grains. Since clumped isotopic composition depends on specific bonding, difference in crystal structure is very likely to affect the way clumped isotopic signatures are recorded in these different structures. This leads to the fact that their pristine signatures might be modified differently by later thermal events like diagenesis/burial heating etc.
Formation
Magnesite can be formed via talc carbonate metasomatism of peridotite and other ultramafic rocks. Magnesite is formed via carbonation of olivine in the presence of water and carbon dioxide at elevated temperatures and high pressures typical of the greenschist facies.
Magnesite can also be formed via the carbonation of magnesium serpentine (lizardite) via the following reaction:
- 2 Mg3Si2O5(OH)4 + 3 CO2 → Mg3Si4O10(OH)2 + 3 MgCO3 + 3 H2O
However, when performing this reaction in the laboratory, the trihydrated form of magnesium carbonate (nesquehonite) will form at room temperature.[17] This very observation led to the postulation of a "dehydration barrier" being involved in the low-temperature formation of anhydrous magnesium carbonate.[18] Laboratory experiments with formamide, a liquid resembling water, have shown how no such dehydration barrier can be involved. The fundamental difficulty to nucleate anhydrous magnesium carbonate remains when using this non-aqueous solution. Not cation dehydration, but rather the spatial configuration of carbonate anions creates the barrier in the low-temperature nucleation of magnesite.[19] Magnesite precipitation needs high pH and absence of other cations.

Magnesite has been found in modern sediments, caves and soils. Its low-temperature (around 40 °C [104 °F]) formation is known to require alternations between precipitation and dissolution intervals.[20][21][22]
Magnesite was detected in meteorite ALH84001 and on planet Mars itself. Magnesite was identified on Mars using infra-red spectroscopy from satellite orbit.[23] Near Jezero Crater, Mg-carbonates have been detected and reported to have formed in lacustrine environment prevailing there.[24] Controversy still exists over the temperature of formation of these carbonates. Low-temperature formation has been suggested for the magnesite from the Mars-derived ALH84001 meteorite.[25][26] The low-temperature formation of magnesite might well be of significance toward large-scale carbon sequestration.[27]
Magnesium-rich olivine (forsterite) favors production of magnesite from peridotite. Iron-rich olivine (fayalite) favors production of magnetite-magnesite-silica compositions.
Magnesite can also be formed by way of metasomatism in skarn deposits, in dolomitic limestones, associated with wollastonite, periclase, and talc.
Resistant to high temperature and able to withstand high pressure, magnesite has been proposed to be one of the major carbonate bearing phase in Earth's mantle.[28] For similar reason, it is found in metamorphosed peridotite rocks in Central Alps, Switzerland [29] and high pressure eclogitic rocks from Tianshan, China.[30]
Magnesite can also precipitate in lakes in presence of bacteria either as hydrous Mg-carbonates or magnesite.[31][32]
Information from Isotopic Structure
Clumped isotopes have been used in interpreting conditions of magnesite formation and the isotopic composition of the precipitating fluid. Within ultramafic complexes, magnesites are found within veins and stockworks in cryptocrystalline form as well as within carbonated peridotite units in crystalline form. These cryptocrystalline forms are mostly variably weathered and yield low temperature of formation.[33] On the other hand, coarse magnesites yield very high temperature indicating hydrothermal origin. It is speculated that coarse high temperature magnesites are formed from mantle derived fluids whereas cryptocrystalline ones are precipitated by circulating meteoric water- taking up carbon from dissolved inorganic carbon pool, soil carbon and affected by disequilibrium isotope effects.
Magnesites forming in lakes and playa settings are in general enriched in heavy isotopes of C and O because of evaporation and CO2 degassing. This reflects in the clumped isotope derived temperature being very low. These are affected by pH effect, biological activity as well as kinetic isotope effect associated with degassing. Magnesite forms as surface moulds in such conditions but more generally occur as hydrous Mg-carbonates since their precipitation is kinetically favored. Most of the times, they derive C from DIC or nearby ultramafic complexes (e.g., Altin Playa, British Columbia, Canada[34]).
Magnesites in metamorphic rocks, on the other hand, indicate very high temperature of formation. Isotopic composition of parental fluid is also heavy- generally metamorphic fluids. This has been verified by fluid inclusion derived temperature as well as traditional O isotope thermometry involving co-precipitating quartz-magnesite.
Often, magnesite records lower clumped isotope temperature than associated dolomite, calcite.[35] The reason might be that calcite, dolomite form earlier at higher temperature (from mantle like fluids) which increases Mg/Ca ratio in the fluid sufficiently so as to precipitate magnesite. As this happens with increasing time, fluid cools, evolves by mixing with other fluids and when it forms magnesite, it decreases its temperature. So the presence of associated carbonates have a control on magnesite isotopic composition.
Origin of Martian carbonates can be deconvolved with the application of clumped isotope. Source of the CO2, climatic-hydrologic conditions on Mars could be assessed from these rocks. Recent study has shown (implementing clumped isotope thermometry) that carbonates in ALH84001 indicate formation at low temperature evaporative condition from subsurface water and derivation of CO2 from Martian atmosphere.[36]
Uses


Similar to the production of lime, magnesite can be burned in the presence of charcoal to produce MgO, which, in the form of a mineral, is known as periclase. Large quantities of magnesite are burnt to make magnesium oxide: an important refractory material used as a lining in blast furnaces, kilns and incinerators. Calcination temperatures determine the reactivity of resulting oxide products and the classifications of light burnt and dead burnt refer to the surface area and resulting reactivity of the product, typically as determined by an industry metric of the iodine number. 'Light burnt' product generally refers to calcination commencing at 450 °C and proceeding to an upper limit of 900 °C – which results in good surface area and reactivity. Above 900 °C, the material loses its reactive crystalline structure and reverts to the chemically inert 'dead-burnt' product- which is preferred for use in refractory materials such as furnace linings.
Magnesite can also be used as a binder in flooring material (magnesite screed).[37] Furthermore, it is being used as a catalyst and filler in the production of synthetic rubber and in the preparation of magnesium chemicals and fertilizers.
In fire assay, magnesite cupels can be used for cupellation as the magnesite cupel will resist the high temperatures involved.
Magnesite can be cut, drilled, and polished to form beads that are used in jewelry-making. Magnesite beads can be dyed into a broad spectrum of bold colors, including a light blue color that mimics the appearance of turquoise.
Research is proceeding to evaluate the practicality of sequestering the greenhouse gas carbon dioxide in magnesite on a large scale.[38] This has focused on peridotites from ophiolites (obducted mantle rocks on crust) where magnesite can be created by letting carbon dioxide react with these rocks. Some progress has been made in ophiolites from Oman.[39] But the major problem is that these artificial processes require sufficient porosity-permeability so that the fluids can flow but this is hardly the case in peridotites.
Occupational safety and health
People can be exposed to magnesite in the workplace by inhaling it, skin contact, and eye contact.
USA
The Occupational Safety and Health Administration (OSHA) has set the legal limit (permissible exposure limit) for magnesite exposure in the workplace as 15 mg/m3 total exposure and 5 mg/m3 respiratory exposure over an 8-hour workday. The National Institute for Occupational Safety and Health (NIOSH) has set a recommended exposure limit (REL) of 10 mg/m3 total exposure and 5 mg/m3 respiratory exposure over an 8-hour workday.[40]
References
- http://rruff.geo.arizona.edu/doclib/hom/magnesite.pdf Handbook of Mineralogy
- http://www.mindat.org/min-2482.html Mindat.org
- http://webmineral.com/data/Magnesite.shtml Webmineral data
- Klein, Cornelis and Cornelius S. Hurlbut, Jr., Manual of Mineralogy, Wiley, 20th ed., p. 332 ISBN 0-471-80580-7
- Ghosh, Prosenjit; Adkins, Jess; Affek, Hagit; Balta, Brian; Guo, Weifu; Schauble, Edwin A.; Schrag, Dan; Eiler, John M. (2006-03-15). "13C–18O bonds in carbonate minerals: A new kind of paleothermometer". Geochimica et Cosmochimica Acta. 70 (6): 1439–1456. doi:10.1016/j.gca.2005.11.014. ISSN 0016-7037.
- Lloyd, Max K.; Ryb, Uri; Eiler, John M. (2018-12-01). "Experimental calibration of clumped isotope reordering in dolomite". Geochimica et Cosmochimica Acta. 242: 1–20. doi:10.1016/j.gca.2018.08.036. ISSN 0016-7037.
- Winkelstern, Ian Z.; Kaczmarek, Stephen E.; Lohmann, Kyger C; Humphrey, John D. (2016-12-02). "Calibration of dolomite clumped isotope thermometry". Chemical Geology. 443: 32–38. doi:10.1016/j.chemgeo.2016.09.021. ISSN 0009-2541.
- Stolper, D. A.; Eiler, J. M. (2015-05-01). "The kinetics of solid-state isotope-exchange reactions for clumped isotopes: A study of inorganic calcites and apatites from natural and experimental samples". American Journal of Science. 315 (5): 363–411. doi:10.2475/05.2015.01. ISSN 0002-9599.
- van Dijk, Joep; Fernandez, Alvaro; Storck, Julian C.; White, Timothy S.; Lever, Mark; Müller, Inigo A.; Bishop, Stewart; Seifert, Reto F.; Driese, Steven G.; Krylov, Alexey; Ludvigson, Gregory A. (June 2019). "Experimental calibration of clumped isotopes in siderite between 8.5 and 62 °C and its application as paleo-thermometer in paleosols". Geochimica et Cosmochimica Acta. 254: 1–20. doi:10.1016/j.gca.2019.03.018. ISSN 0016-7037.
- Stolper, D. A.; Lawson, M.; Davis, C. L.; Ferreira, A. A.; Neto, E. V. Santos; Ellis, G. S.; Lewan, M. D.; Martini, A. M.; Tang, Y.; Schoell, M.; Sessions, A. L. (2014-06-27). "Formation temperatures of thermogenic and biogenic methane". Science. 344 (6191): 1500–1503. doi:10.1126/science.1254509. ISSN 0036-8075. PMID 24970083.
- Yeung, Laurence Y.; Young, Edward D.; Schauble, Edwin A. (2012). "Measurements of 18O18O and 17O18O in the atmosphere and the role of isotope-exchange reactions". Journal of Geophysical Research: Atmospheres. 117 (D18). doi:10.1029/2012JD017992. ISSN 2156-2202.
- Śliwiński, Maciej G.; Kitajima, Kouki; Spicuzza, Michael J.; Orland, Ian J.; Ishida, Akizumi; Fournelle, John H.; Valley, John W. (2017-11-22). "SIMS Bias on Isotope Ratios in Ca-Mg-Fe Carbonates (Part III): δ18O and δ13C Matrix Effects Along the Magnesite-Siderite Solid-Solution Series". Geostandards and Geoanalytical Research. 42 (1): 49–76. doi:10.1111/ggr.12194. ISSN 1639-4488.
- García del Real, Pablo; Maher, Kate; Kluge, Tobias; Bird, Dennis K.; Brown, Gordon E.; John, Cédric M. (November 2016). "Clumped-isotope thermometry of magnesium carbonates in ultramafic rocks". Geochimica et Cosmochimica Acta. 193: 222–250. doi:10.1016/j.gca.2016.08.003. ISSN 0016-7037.
- Sharma, S.Das; Patil, D.J; Gopalan, K (February 2002). "Temperature dependence of oxygen isotope fractionation of CO 2 from magnesite-phosphoric acid reaction". Geochimica et Cosmochimica Acta. 66 (4): 589–593. doi:10.1016/s0016-7037(01)00833-x. ISSN 0016-7037.
- Rosenbaum, J; Sheppard, S.M.F (June 1986). "An isotopic study of siderites, dolomites and ankerites at high temperatures". Geochimica et Cosmochimica Acta. 50 (6): 1147–1150. doi:10.1016/0016-7037(86)90396-0. ISSN 0016-7037.
- Guo, Weifu (January 2020). "Kinetic clumped isotope fractionation in the DIC-H2O-CO2 system: Patterns, controls, and implications". Geochimica et Cosmochimica Acta. 268: 230–257. doi:10.1016/j.gca.2019.07.055.
- Leitmeier, H.(1916): Einige Bemerkungen über die Entstehung von Magnesit und Sideritlagerstätten, Mitteilungen der Geologischen Gesellschaft in Wien, vol.9, pp. 159–166.
- Lippmann, F. (1973): Sedimentary carbonate minerals. Springer Verlag, Berlin, 228 p.
- Xu, J; Yan, C.; Zhang, F.; Konishi, H., Xu, H. & Teng, H. H. (2013): Testing the cation-hydration effect on the crystallization of Ca – Mg- CO3 systems. Proc. Natl. Acad. Sci. US, vol.110 (44), pp.17750-17755.
- Deelman, J.C. (1999): "Low-temperature nucleation of magnesite and dolomite", Neues Jahrbuch für Mineralogie, Monatshefte, pp. 289–302.
- Alves dos Anjos et al. (2011): Synthesis of magnesite at low temperature. Carbonates and Evaporites, vol.26, pp.213-215.
- Hobbs, F. W. C. and Xu, H. (2020): Magnesite formation through temperature and pH cycling as a proxy for lagoon and playa environments. Geochimica et Cosmochimica Acta, vol.269, pp.101-116.
- Ehlmann, B. L. et al. (2008): Orbital identification of carbonate-bearing rocks on Mars. Science, vol.322, no.5909, pp.1828-1832.
- Horgan, Briony H.N.; Anderson, Ryan B.; Dromart, Gilles; Amador, Elena S.; Rice, Melissa S. (March 2020). "The mineral diversity of Jezero crater: Evidence for possible lacustrine carbonates on Mars". Icarus. 339: 113526. doi:10.1016/j.icarus.2019.113526. ISSN 0019-1035.
- McSween Jr, H. Y and Harvey, R. P.(1998): An evaporation model for formation of carbonates in the ALH84001 Martian meteorite. International Geology Review, vol.49, pp.774-783.
- Warren, P. H. (1998): Petrologic evidence for low-temperature, possibly flood evaporitic origin of carbonates in the ALH84001 meteorite. Journal of Geophysical Research, vol.103, no.E7, 16759-16773.
- Oelkers, E. H.; Gislason, S. R. and Matter, J. (2008): Mineral carbonation of CO2. Elements, vol.4, pp.333-337.
- Isshiki, Maiko; Irifune, Tetsuo; Hirose, Kei; Ono, Shigeaki; Ohishi, Yasuo; Watanuki, Tetsu; Nishibori, Eiji; Takata, Masaki; Sakata, Makoto (January 2004). "Stability of magnesite and its high-pressure form in the lowermost mantle". Nature. 427 (6969): 60–63. doi:10.1038/nature02181. ISSN 0028-0836.
- FERRY, JOHN M.; RUMBLE, DOUGLAS; WING, BOSWELL A.; PENNISTON-DORLAND, SARAH C. (2005-04-22). "A New Interpretation of Centimetre-scale Variations in the Progress of Infiltration-driven Metamorphic Reactions: Case Study of Carbonated Metaperidotite, Val d'Efra, Central Alps, Switzerland". Journal of Petrology. 46 (8): 1725–1746. doi:10.1093/petrology/egi034. ISSN 1460-2415.
- Zhang, Lifei; Ellis, David J.; Williams, Samantha; Jiang, Wenbo (July 2002). "Ultra-high pressure metamorphism in western Tianshan, China: Part II. Evidence from magnesite in eclogite". American Mineralogist. 87 (7): 861–866. doi:10.2138/am-2002-0708. ISSN 0003-004X.
- Mavromatis, Vasileios; Pearce, Christopher R.; Shirokova, Liudmila S.; Bundeleva, Irina A.; Pokrovsky, Oleg S.; Benezeth, Pascale; Oelkers, Eric H. (2012-01-01). "Magnesium isotope fractionation during hydrous magnesium carbonate precipitation with and without cyanobacteria". Geochimica et Cosmochimica Acta. 76: 161–174. doi:10.1016/j.gca.2011.10.019. ISSN 0016-7037.
- Shirokova, Liudmila S.; Mavromatis, Vasileios; Bundeleva, Irina A.; Pokrovsky, Oleg S.; Bénézeth, Pascale; Gérard, Emmanuelle; Pearce, Christopher R.; Oelkers, Eric H. (2013-01-01). "Using Mg Isotopes to Trace Cyanobacterially Mediated Magnesium Carbonate Precipitation in Alkaline Lakes". Aquatic Geochemistry. 19 (1): 1–24. doi:10.1007/s10498-012-9174-3. ISSN 1573-1421.
- Quesnel, Benoît; Boulvais, Philippe; Gautier, Pierre; Cathelineau, Michel; John, Cédric M.; Dierick, Malorie; Agrinier, Pierre; Drouillet, Maxime (June 2016). "Paired stable isotopes (O, C) and clumped isotope thermometry of magnesite and silica veins in the New Caledonia Peridotite Nappe". Geochimica et Cosmochimica Acta. 183: 234–249. doi:10.1016/j.gca.2016.03.021. ISSN 0016-7037.
- Power, Ian M.; Harrison, Anna L.; Dipple, Gregory M.; Wilson, Siobhan A.; Barker, Shaun L.L.; Fallon, Stewart J. (June 2019). "Magnesite formation in playa environments near Atlin, British Columbia, Canada". Geochimica et Cosmochimica Acta. 255: 1–24. doi:10.1016/j.gca.2019.04.008. ISSN 0016-7037.
- Streit, Elisabeth; Kelemen, Peter; Eiler, John (2012-06-17). "Coexisting serpentine and quartz from carbonate-bearing serpentinized peridotite in the Samail Ophiolite, Oman". Contributions to Mineralogy and Petrology. 164 (5): 821–837. doi:10.1007/s00410-012-0775-z. ISSN 0010-7999.
- Halevy, Itay; Fischer, Woodward W.; Eiler, John M. (2011-10-11). "Carbonates in the Martian meteorite Allan Hills 84001 formed at 18 ± 4 °C in a near-surface aqueous environment". Proceedings of the National Academy of Sciences. 108 (41): 16895–16899. doi:10.1073/pnas.1109444108. ISSN 0027-8424. PMC 3193235. PMID 21969543.
- Information about magnesite flooring, West Coast Deck Water Proofing
- "Scientists find way to make mineral which can remove CO2 from atmosphere". phys.org/news. Retrieved 2018-08-15.
- Kelemen, Peter B.; Matter, Juerg; Streit, Elisabeth E.; Rudge, John F.; Curry, William B.; Blusztajn, Jerzy (2011-05-30). "Rates and Mechanisms of Mineral Carbonation in Peridotite: Natural Processes and Recipes for Enhanced, in situ CO2Capture and Storage". Annual Review of Earth and Planetary Sciences. 39 (1): 545–576. doi:10.1146/annurev-earth-092010-152509. ISSN 0084-6597.
- "CDC – NIOSH Pocket Guide to Chemical Hazards – Magnesite". www.cdc.gov. Retrieved 2015-11-19.
- Smithsonian Rock and Gem ISBN 0-7566-0962-3