Lysis
Lysis (/ˈlaɪsɪs/ LY-sis; Greek λύσις lýsis, "a loosing" from λύειν lýein, "to unbind") is the breaking down of the membrane of a cell, often by viral, enzymic, or osmotic (that is, "lytic" /ˈlɪtɪk/ LIT-ək) mechanisms that compromise its integrity. A fluid containing the contents of lysed cells is called a lysate. In molecular biology, biochemistry, and cell biology laboratories, cell cultures may be subjected to lysis in the process of purifying their components, as in protein purification, DNA extraction, RNA extraction, or in purifying organelles.
Many species of bacteria are subject to lysis by the enzyme lysozyme, found in animal saliva, egg white, and other secretions.[1] Phage lytic enzymes (lysins) produced during bacteriophage infection are responsible for the ability of these viruses to lyse bacterial cells.[2] Penicillin and related β-lactam antibiotics cause the death of bacteria through enzyme-mediated lysis that occurs after the drug causes the bacterium to form a defective cell wall.[3] If the cell wall is completely lost and the penicillin was used on gram-positive bacteria, then the bacterium is referred to as a protoplast, but if penicillin was used on gram-negative bacteria, then it is called a spheroplast.
Cytolysis
Cytolysis occurs when a cell bursts due to an osmotic imbalance that has caused excess water to move into the cell.
Cytolysis can be prevented by several different mechanisms, including the contractile vacuole that exists in some paramecia, which rapidly pump water out of the cell. Cytolysis does not occur under normal conditions in plant cells because plant cells have a strong cell wall that contains the osmotic pressure, or turgor pressure, that would otherwise cause cytolysis to occur.
Oncolysis
Oncolysis refers to the destruction of neoplastic cells or of a tumour.
It is also used to refer to the reduction of any swelling.[4]
Plasmolysis
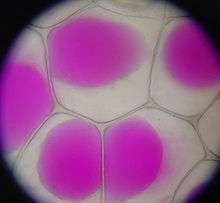
Plasmolysis is the contraction of cells within plants due to the loss of water through osmosis. In a hypertonic environment, the cell membrane peels off of the cell wall and the vacuole collapses. These cells will eventually wilt and die unless the flow of water caused by osmosis can stop the contraction of the cell membrane.[5]
Immune response
Erythrocytes' hemoglobin release free radicals in response to pathogens when lysed by them. This can damage the pathogens.[6][7]
Applications
Cell lysis is used in laboratories to break open cells and purify or further study their contents. Lysis in the laboratory may be affected by enzymes or detergents or other chaotropic agents. Mechanical disruption of cell membranes, as by repeated freezing and thawing, sonication, pressure, or filtration may also be referred to as lysis. Many laboratory experiments are sensitive to the choice of lysis mechanism; often it is desirable to avoid mechanical shear forces that would denature or degrade sensitive macromolecules, such as proteins and DNA, and different types of detergents can yield different results. The unprocessed solution immediately after lysis but before any further extraction steps is often referred to as a crude lysate.[8][9]
For example, lysis is used in western and Southern blotting to analyze the composition of specific proteins, lipids, and nucleic acids individually or as complexes. Depending on the detergent used, either all or some membranes are lysed. For example, if only the cell membrane is lysed then gradient centrifugation can be used to collect certain organelles. Lysis is also used for protein purification, DNA extraction, and RNA extraction.[8][9]
Methods
Electrochemical Lysis
This method uses hydroxide ions which are created electrochemically within the device by a palladium electrode, porating the membrane of a cell causing cell lysis. The advantages of this method include selective lysing.[10]
Chemical Lysis
This method uses chemical disruption. It is the most popular and simple approach. Chemical lysis chemically deteriorate/solubilize the proteins and lipids present within the membrane of targeted cells.[11]
Acoustic Lysis
This method uses ultrasonic waves to generate areas of high and low pressure which causes cavitation and in turn, cell lysis. Though this method usually comes out clean, it fails to be cost effective and consistent.[12]
Mechanical Lysis
This method uses physical penetration to pierce or cut a cell membrane.[13]
References
- P. Jollès, ed. (1996). Lysozymes--model enzymes in biochemistry and biology. Basel: Birkhäuser Verlag. pp. 35–64. ISBN 978-3-7643-5121-2.
- Nelson, D.; Loomis, L.; Fischetti, V. A. (20 March 2001). "Prevention and elimination of upper respiratory colonization of mice by group A streptococci by using a bacteriophage lytic enzyme". Proceedings of the National Academy of Sciences. 98 (7): 4107–12. Bibcode:2001PNAS...98.4107N. doi:10.1073/pnas.061038398. PMC 31187. PMID 11259652.
- Scholar, E. M.; Pratt, W. B. (2000). The antimicrobial drugs (2nd ed.). Oxford University Press. pp. 61–64.
- "Oncolysis". Medical Dictionary. Farlex. Retrieved 27 March 2013.
- "Wiley InterScience : Journals : New Phytologist". New Phytologist. 126: 571–591. doi:10.1111/j.1469-8137.1994.tb02952.x. Archived from the original on May 22, 2011. Retrieved 2008-09-11.
- Red blood cells do more than just carry oxygen. New findings by NUS team show they aggressively attack bacteria too. Archived 2009-02-20 at the Wayback Machine, The Straits Times, 1 September 2007
- Jiang N, Tan NS, Ho B, Ding JL; Tan; Ho; Ding (October 2007). "Respiratory protein-generated reactive oxygen species as an antimicrobial strategy". Nature Immunology. 8 (10): 1114–22. doi:10.1038/ni1501. PMID 17721536.CS1 maint: multiple names: authors list (link)
- Thermo Scientific Pierce Cell Lysis Technical Handbook (PDF) (2 ed.). Thermo Scientific.
- "Protein Expression and Purification Core Facility: Protein Purification: Extraction and Clarification". European Molecular Biology Laboratory. Retrieved 17 March 2015.
- Park, Seung-min; Sabour, Andrew F; Ho Son, Jun; Hun Lee, Sang; Lee, Luke (2014). "Toward Integrated Molecular Diagnostic System (iMDx): Principles and Applications". IEEE Transactions on Bio-Medical Engineering. 61 (5): 1506–1521. doi:10.1109/TBME.2014.2309119. PMC 4141683. PMID 24759281.
- Park, Seung-min; Sabour, Andrew F; Ho Son, Jun; Hun Lee, Sang; Lee, Luke (2014). "Toward Integrated Molecular Diagnostic System (iMDx): Principles and Applications". IEEE Transactions on Bio-Medical Engineering. 61 (5): 1506–1521. doi:10.1109/TBME.2014.2309119. PMC 4141683. PMID 24759281.
- Park, Seung-min; Sabour, Andrew F; Ho Son, Jun; Hun Lee, Sang; Lee, Luke (2014). "Toward Integrated Molecular Diagnostic System (iMDx): Principles and Applications". IEEE Transactions on Bio-Medical Engineering. 61 (5): 1506–1521. doi:10.1109/TBME.2014.2309119. PMC 4141683. PMID 24759281.
- Park, Seung-min; Sabour, Andrew F; Ho Son, Jun; Hun Lee, Sang; Lee, Luke (2014). "Toward Integrated Molecular Diagnostic System (iMDx): Principles and Applications". IEEE Transactions on Bio-Medical Engineering. 61 (5): 1506–1521. doi:10.1109/TBME.2014.2309119. PMC 4141683. PMID 24759281.