Lignosulfonates
Lignosulfonates, or sulfonated lignin are water-soluble anionic polyelectrolyte polymers: they are byproducts from the production of wood pulp using sulfite pulping.[1]
Most delignification in sulfite pulping involves acidic cleavage of ether bonds, which connect many of the constituents of lignin.[2] The electrophilic carbocations produced during ether cleavage react with bisulfite ions (HSO3−) to give sulfonates.
- R-O-R' + H+ → R+ + R'OH
- R+ + HSO3− → R-SO3H
The primary site for ether cleavage is the α-carbon (carbon atom attached to the aromatic ring) of the propyl (linear three carbon) side chain. The following structures do not specify the structure since lignin and its derivatives are complex mixtures: the purpose is to give a general idea of the structure of lignosulfonates. The groups R1 and R2 can be a wide variety of groups found in the structure of lignin. Sulfonation occurs on the side chains, not on the aromatic ring like in p-toluenesulfonic acid.
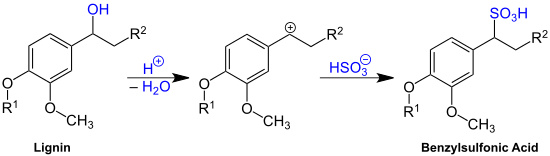
Lignosulfonate have very broad ranges of molecular mass (they are very polydisperse). A range of from 1000–140,000 da has been reported for softwood lignosulfonates with lower values reported for hardwoods.[1]
Preparation
Lignosulfonates are recovered from the spent pulping liquids (red or brown liquor) from sulfite pulping. Ultrafiltration can also be used to separate lignosulfonates from the spent pulping liquid.[1] A list of CAS numbers for the various metal salts of lignosulfonate is available.[3]
Uses
Lignosulfonates have a wide variety of applications.
The single largest use for lignosulfonates is as plasticizers in making concrete,[1] where they allow concrete to be made with less water (giving stronger concrete) while maintaining the ability of the concrete to flow. Lignosulfonates are also used during the production of cement, where they act as grinding aids in the cement mill and as a rawmix slurry deflocculant (that reduces the viscosity of the slurry).
Lignosulfonates are also used for the production of plasterboard to reduce the amount of water required to make the stucco flow and form the layer between two sheets of paper. The reduction in water content allows lower kiln temperatures to dry the plasterboard, saving energy.
The ability of lignosulfonates to reduce the viscosity of mineral slurries is used to advantage in oil drilling mud, where it replaced tannic acids from quebracho (a tropical tree).
Lignosulfonates are used to disperse pesticides, dyes, carbon black, and other insoluble solids and liquids into water. They are used in tanning leather. They are also used to suppress dust on unpaved roads.
Oxidation of lignosulfonates from softwood trees produced vanillin (artificial vanilla flavor).
Dimethyl sulfide and dimethyl sulfoxide (an important organic solvent) are produced from lignosulfonates. The first step involves heating lignosulfonates with sulfides or elemental sulfur to produce dimethyl sulfide. The methyl groups come from methyl ethers present in the lignin. Oxidation of dimethyl sulfide with nitrogen dioxide produces dimethyl sulfoxide (DMSO). [1]
Also one of the very wide uses of lignosulfonates is deflocculation of clays used in drilling fluids in the oil and gas industry. Furthermore, Lignosulphates are being researched for use in Enhanced oil recovery (EOR) due to their ability to reduce IFT in foams, allowing for improved sweep efficiency, and hence increased recovery factor.
Aqueous Lignosulfonate solutions are also widely used as a non-toxic dust suppression agent for unpaved road surfaces, where it is popularly, if erroneously, called "tree sap". Roads treated with lignosulfonates can be distinguished from those treated with calcium chloride by color: lignosulfonates give the road surface a dark grey color, while calcium chloride lend the road surface a distinctive tan or brown color. As lignosulfonates do not rely on water to provide their binding properties, they tend to be more useful in arid locations.
They also form a constituent of the paste used to coat the lead-antimony-calcium or lead-antimony-selenium grids in a Lead-acid battery.
Besides their use as dispersants lignosulfonates are also good binders. They are used as binders in well-paper, particle boards, linoleum flooring, coal briquettes, and roads.
The anti-oxidant effect of lignosulfonates is utilized in feeds, ensilage and flame retardants.
The UV absorbance of lignosulfonates is utilized in sun screens and bio-pesticides.
References
- Lebo, Stuart E. Jr.; Gargulak, Jerry D.; McNally, Timothy J. (2001). "Lignin". Kirk-Othmer Encyclopedia of Chemical Technology. Kirk‑Othmer Encyclopedia of Chemical Technology. John Wiley & Sons, Inc. doi:10.1002/0471238961.12090714120914.a01.pub2. ISBN 0-471-23896-1. Retrieved 2007-10-14.
- E. Sjöström (1993). Wood Chemistry: Fundamentals and Applications. Academic Press.
- "List of lignosulfonate CAS numbers". Retrieved 2007-10-15.