Kostanecki acylation
The Kostanecki acylation is a method used in organic synthesis to form chromones[1] or coumarins[2] by acylation of O-hydroxyaryl ketones with aliphatic acid anhydrides, followed by cyclization.[3] If benzoic anhydride (or benzoyl chloride) is used, a particular type of chromone called a flavone is obtained.
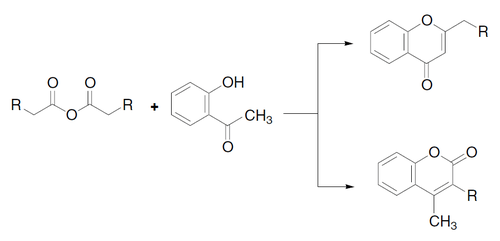
Mechanism
The mechanism consists of three well-differentiated reactions:[4][5]
- Phenol O-acylation with formation of a tetrahedral intermediate
- Intramolecular aldol condensation to cyclize and to form a hydroxydihydrochromone
- Elimination of the hydroxyl group to form the chromone (or coumarin)
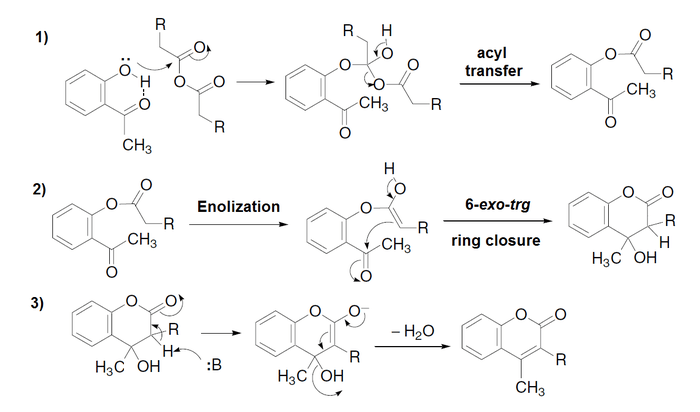
Examples
gollark: ++help
gollark: ?emojistats
gollark: ++help
gollark: I know, this is hard.
gollark: Hmm, should I just put everything in docker, or...?
References
- Gaspar, A.; Matos, M. J. O.; Garrido, J.; Uriarte, E.; Borges, F. (2014). "Chromone: A Valid Scaffold in Medicinal Chemistry". Chemical Reviews. 114 (9): 4960–92. doi:10.1021/cr400265z. PMID 24555663.
- Sethna, S. M.; Shah, N. M. (1945). "The Chemistry of Coumarins". Chemical Reviews. 36: 1–62. doi:10.1021/cr60113a001.
- v. Kostanecki, St.; Różycki, A. (1901). "Ueber eine Bildungsweise von Chromonderivaten". Berichte der Deutschen Chemischen Gesellschaft. 34: 102–109. doi:10.1002/cber.19010340119.
- Mamedov, V. A.; Kalinin, A. A.; Gubaidullin, A. T.; Litvinov, I. A. (2003). Chemistry of Heterocyclic Compounds. 39: 96–100. doi:10.1023/A:1023028927007. Missing or empty
|title=(help) - Ellis, G. P. (1977) Chromenes, Chromanones, and Chromones from The Chemistry of Heterocyclic Compounds, Weissberger, A. and Taylor, E. C., eds.; Wiley & Sons: New York, vol. 31, p. 495.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.