Allan–Robinson reaction
The Allan–Robinson reaction is the chemical reaction of o-hydroxyaryl ketones with aromatic anhydrides to form flavones (or isoflavones).[1][2][3][4]
If aliphatic anhydrides are used, coumarins can also be formed. (See Kostanecki acylation)
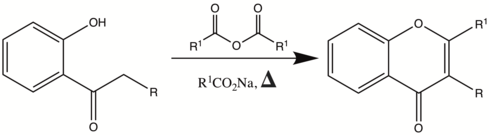 Reaction Overview
Reaction Overview
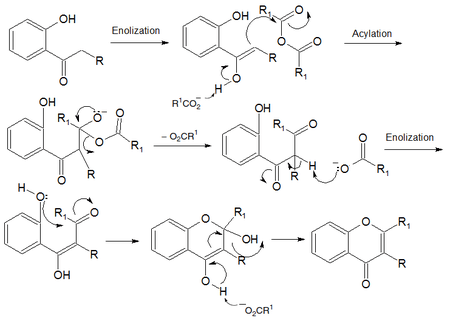
Mechanism
The first step is an enolization which is a proton transfer created a hydroxide instead of a carbonyl and an alkene instead of an alkane. The second step is an acylation in which the newly formed bond from the enolization attacks an electrophilic carbon in the anhydride. The third step displays the carboxylate functionality leaving since it is the best leaving group. As a result the resulting carboxylate attacks an alpha hydrogen to create the enol functionality again in step four. The fifth step shows the nucleophilic hydroxide attacking the carbonyl carbon to create a new six membered heterocyclic ring. The resulting structure undergoes a proton transfer in step 6 to achieve the final product. All six of these steps occur in the reaction flask in the presence of heat.[4][5]
References
- Allan, J.; Robinson, R. J. Chem. Soc. 1924, 125, 2192.
- Dyke, S. F.; Ollis, W. D.; Sainsbury, M. J. Org. Chem. 1961, 26, 2453. (doi:10.1021/jo01351a072)
- Wheller, T. S. (1952). "Flavone". Organic Syntheses. 32: 72. doi:10.15227/orgsyn.032.0072.
- Jie Jack Li (2009). Name Reactions. A Collection of Detailed Reaction Mechanisms 4th Edition. Berlin, De: Springer. ISBN 978-3-642-01052-1.
- Graham, Solomons, T. W. (17 January 2013). Organic chemistry. Fryhle, Craig B.,, Snyder, S. A. (Scott A.) (11e ed.). Hoboken, NJ. ISBN 9781118147399. OCLC 820665397.