Isoxazole
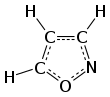
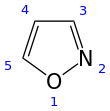
Isoxazole is an azole with an oxygen atom next to the nitrogen. It is also the class of compounds containing this ring. Isoxazolyl is the univalent radical derived from isoxazole.
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
1,2-oxazole | |||
| Other names
isoxazole | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.005.472 | ||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C3H3NO | |||
| Molar mass | 69.06202 g/mol | ||
| Density | 1.075 g/ml | ||
| Boiling point | 95 °C (203 °F; 368 K) | ||
| Acidity (pKa) | -3.0 (of conjugate acid) [1] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Occurrence
Isoxazole rings are found in some natural products, such as ibotenic acid and muscimol.
Pharmaceuticals and herbicides
Isoxazoles also form the basis for a number of drugs,[2] including the COX-2 inhibitor valdecoxib (Bextra) and a neurotransmitter agonist AMPA. A derivative, furoxan, is a nitric oxide donor. An isoxazolyl group is found in many beta-lactamase-resistant antibiotics, such as cloxacillin, dicloxacillin and flucloxacillin. Leflunomide is an isoxazole-derivative drug. Examples of AAS containing the isoxazole ring include danazol and androisoxazole. A number of pesticides are isoxazoles.[3]
See also
References
- Zoltewicz, J. A. & Deady, L. W. Quaternization of heteroaromatic compounds. Quantitative aspects. Adv. Heterocycl. Chem. 22, 71-121 (1978).
- Zhu, Jie; Mo, Jun; Lin, Hong-zhi; Chen, Yao; Sun, Hao-peng (2018). "The recent progress of isoxazole in medicinal chemistry". Bioorganic & Medicinal Chemistry. 26 (12): 3065–3075. doi:10.1016/j.bmc.2018.05.013.CS1 maint: uses authors parameter (link)
- Clemens Lamberth (2018). "Oxazole and Isoxazole Chemistry in Crop Protection". Journal of Heterocyclic Chemistry. 55 (9): 2035–2045. doi:10.1002/jhet.3252.