IMes
IMes is an abbreviation for an organic compound that is a common ligand in organometallic chemistry. It is an N-heterocyclic carbene (NHC). The compound, a white solid, is often not isolated but instead is generated upon attachment to the metal centre.[1]
imidazol-2-ylidene_(aka_IMes).png) | |
| Names | |
|---|---|
| Other names
1,3-Dimesitylimidazol-2-ylidene, 1,3-bis(2,4,6-trimethylphenyl)-imidazolium, 1,3-bis(2,4,6-trimethylphenyl)imidazol-2-ylidene | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.154.201 |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C21H24N2 | |
| Molar mass | 304.43 |
| Appearance | white solid |
| Melting point | 150 to 155 °C (302 to 311 °F; 423 to 428 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
First prepared by Arduengo,[2] the heterocycle is synthesized by condensation of 2,4,6-trimethylaniline and glyoxal to give the diimine. In the presence of acid, this diimine condenses with formaldehyde to give the dimesitylimidazolium cation. This cation is the conjugate acid of the NHC.[3][4]
Related compounds
Bulkier than IMes is the NHC ligand IPr (CAS RN 244187-81-3). IPr features diisopropylphenyl in place of the mesityl substituents.[5]
Some variants of IMes and IPr have saturated backbones, two such ligands are SIMes and SIPr.[1] They are prepared by alkylation of substituted anilines with dibromoethane followed by ring closure and dehydrohalogenation of the dihydroimidazolium salt.[6]
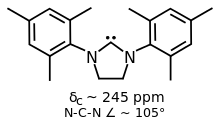 SIMes is a popular NHC ligand with a more flexible backbone compared to IMes
SIMes is a popular NHC ligand with a more flexible backbone compared to IMes
References
- Steven P. Nolan "N-Heterocyclic Carbenes in Synthesis" Wiley-VCH, 2006. ISBN 978-3-527-60940-6.
- Arduengo, Anthony J., III; Dias, H. V. Rasika; Harlow, Richard L.; Kline, Michael "Electronic Stabilization of Nucleophilic Carbenes" Journal of the American Chemical Society 1992, volume 114, pp. 5530-4. doi:10.1021/ja00040a007
- Elon A. Ison, Ana Ison "Synthesis of Well-Defined Copper N-Heterocyclic Carbene Complexes and Their Use as Catalysts for a “Click Reaction”: A Multistep Experiment That Emphasizes the Role of Catalysis in Green Chemistry" J. Chem. Educ., 2012, volume 89, pp 1575–1577. doi:10.1021/ed300243s
- Chen, Junting; Ritter, Tobias (2019). "Late-Stage Deoxyfluorination of Phenols with PhenoFluorMix". Org. Synth. 96: 16. doi:10.15227/orgsyn.096.0016.
- Morgan Hans; Lionel Delaude (2010). "Microwave-Assisted Synthesis of 1,3-Dimesitylimidazolium Chloride". Org. Synth. 87: 77. doi:10.15227/orgsyn.087.0077.
- Arnaud Gautier, Federico Cisnetti, Silvia Díez-González, Clémentine Gibard "Synthesis of 1,3–bis(2,4,6–trimethylphenyl)–imidazolinium salts : SIMes.HCl, SIMes.HBr, SIMes.HBF4 and SIMes.HPF6" Protocol Exchange 2012. doi:10.1038/protex.2012.048. http://www.nature.com/protocolexchange/protocols/2488#/procedure
Further reading
- Xavier Bantreil & Steven P. Nolan "Synthesis of N-heterocyclic carbene ligands and derived ruthenium olefin metathesis catalysts" Nature Protocols 2011, volume 6, 69–77. doi:10.1038/nprot.2010.177.