Hyperpolarized carbon-13 MRI
Hyperpolarized carbon-13 MRI is a functional medical imaging technique for probing perfusion and metabolism using injected substrates.
| Hyperpolarized carbon-13 MRI | |
|---|---|
| Purpose | imaging technique for probing perfusion and metabolism |
It is enabled by techniques for hyperpolarization of carbon-13-containing molecules using dynamic nuclear polarization and rapid dissolution to create an injectable solution.[1][2] Following the injection of a hyperpolarized substrate, metabolic activity can be mapped based on enzymatic conversion of the injected molecule. In contrast with other metabolic imaging methods such as positron emission tomography, hyperpolarized carbon-13 MRI provides chemical as well as spatial information, allowing this technique to be used to probe the activity of specific metabolic pathways. This has led to new ways of imaging disease. For example, metabolic conversion of hyperpolarized pyruvate into lactate is increasingly being used to image cancerous tissues via the Warburg effect.[3][4][5]
Hyperpolarization
While hyperpolarization of inorganic small molecules (like 3He and 129Xe) is generally achieved using spin-exchange optical pumping (SEOP), compounds useful for metabolic imaging (such as 13C or 15N) are typically hyperpolarized using dynamic nuclear polarization (DNP). DNP can be performed at operating temperatures of 1.1-1.2 K, and high magnetic fields (~4T).[6] The compounds are then thawed and dissolved to yield a room temperature solution containing hyperpolarized nuclei which can be injected.
Dissolution and injection
Hyperpolarized samples of 13C pyruvic acid are typically dissolved in some form of aqueous solution containing various detergents and buffering reagents. For example, in a study detecting tumor response to etoposide treatment, the sample was dissolved in 40 mM HEPES, 94 mM NaOH, 30 mM NaCl, and 50 mg/L EDTA.[3]
Preclinical models
Hyperpolarzied carbon-13 MRI is currently being developed as a potentially cost effective diagnostic and treatment progress tool in various cancers, including prostate cancer. Other potential uses include neuro-oncological applications such as the monitoring of real-time in vivo metabolic events.[7]
Clinical trials
The majority of clinical studies utilizing 13C hyperpolarization are currently studying pyruvate metabolism in prostate cancer, testing reproducibility of the imaging data, as well as feasibility of acquiring time.[8]
Imaging methods
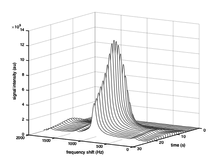
Spectroscopic imaging
Spectroscopic imaging techniques enable chemical information to be extracted from hyperpolarized carbon-13 MRI experiments. The distinct chemical shift associated with each metabolite can be exploited to probe the exchange of magnetization between pools corresponding to each of the metabolites.
Metabolite-selective excitation
Using techniques for simultaneous spatial and spectral selective excitation, RF pulses can be designed to perturb metabolites individually.[9][10] This enables the encoding of metabolite-selective images without the need for spectroscopic imaging. This technique also allows different flip angles to be applied to each metabolite,[11][12] which enables pulse sequences to be designed that make optimal use of the limited polarization available for imaging.[13][14]
Dynamic imaging models
In contrast with conventional MRI, hyperpolarized experiments are inherently dynamic as images must be acquired as the injected substrate spreads through the body and is metabolized. This necessitates dynamical system modelling and estimation for quantifying metabolic reaction rates. A number of approaches exist for modeling the evolution of magnetization within a single voxel.
| pyruvate | lactate | alanine | |
|---|---|---|---|
| T1 | ~46.9-65 s dependent on B0 field strength[15] | ||
| T2 (HCC Tumor) | 0.9 ± 0.2 s[16] | 1.2 ± 0.1 s[16] | |
| T2 (Healthy Liver) | 0.52 ± 0.03 s[16] | 0.38 ± 0.05 s[16] |
Two-species model with unidirectional flux
The simplest model of metabolic flux assumes unidirectional conversion of the injected substrate S to a product P. The rate of conversion is assumed to be governed by the reaction rate constant
-
.
(1)
Exchange of magnetization between the two species can then be modeled using the linear ordinary differential equation
where denotes the rate at which the transverse magnetization decays to thermal equilibrium polarization, for the product species P.
Two-species model with bidirectional flux
The unidirectional flux model can be extended to account for bidirectional metabolic flux with forward rate and backward rate
-
(2)
The differential equation describing the magnetization exchange is then
Effect of radio-frequency excitation
Repeated radio-frequency (RF) excitation of the sample causes additional decay of the magnetization vector. For constant flip angle sequences, this effect can be approximated using a larger effective rate of decay computed as
where is the flip angle and is the repetition time.[17] Time-varying flip angle sequences can also be used, but require that the dynamics be modeled as a hybrid system with discrete jumps in the system state.[18]
Metabolism mapping
The goal of many hyperpolarized carbon-13 MRI experiments is to map the activity of a particular metabolic pathway. Methods of quantifying the metabolic rate from dynamic image data include temporally integrating the metabolic curves, computing the definite integral referred to in pharmacokinetics as the area under the curve (AUC), and taking the ratio of integrals as a proxy for rate constants of interest.
Area-under-the-curve ratio
Comparing the definite integral under the substrate and product metabolite curves has been proposed as an alternative to model-based parameter estimates as a method of quantifying metabolic activity. Under specific assumptions, the ratio
of area under the product curve AUC(P) to the area under the substrate curve AUC(S) is proportional to the forward metabolic rate .[19]
Rate parameter mapping
When the assumptions under which this ratio is proportional to are not met, or there is significant noise in the collected data, it is desirable to compute estimates of model parameters directly. When noise is independent and identically distributed and Gaussian, parameters can be fit using non-linear least squares estimation. Otherwise (for example if magnitude images with Rician-distributed noise are used), parameters can be estimated by maximum likelihood estimation. The spatial distribution of metabolic rates can be visualized by estimating metabolic rates corresponding to the time series from each voxel, and plotting a heat map of the estimated rates.
See also
References
- Ardenkjaer-Larsen JH, Fridlund B, Gram A, Hansson G, Hansson L, Lerche MH, Servin R, Thaning M, Golman K (September 2003). "Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR". Proceedings of the National Academy of Sciences of the United States of America. 100 (18): 10158–63. Bibcode:2003PNAS..10010158A. doi:10.1073/pnas.1733835100. PMC 193532. PMID 12930897.
- Golman K, Ardenkjaer-Larsen JH, Petersson JS, Mansson S, Leunbach I (September 2003). "Molecular imaging with endogenous substances". Proceedings of the National Academy of Sciences of the United States of America. 100 (18): 10435–9. Bibcode:2003PNAS..10010435G. doi:10.1073/pnas.1733836100. PMC 193579. PMID 12930896.
- Day SE, Kettunen MI, Gallagher FA, Hu DE, Lerche M, Wolber J, Golman K, Ardenkjaer-Larsen JH, Brindle KM (November 2007). "Detecting tumor response to treatment using hyperpolarized 13C magnetic resonance imaging and spectroscopy". Nature Medicine. 13 (11): 1382–7. doi:10.1038/nm1650. PMID 17965722.
- Sriram R, Kurhanewicz J, Vigneron DB (2014). "Hyperpolarized Carbon-13 MRI and MRS Studies". eMagRes. 3: 1–14. doi:10.1002/9780470034590.emrstm1253.
- Nelson SJ, Kurhanewicz J, Vigneron DB, Larson PE, Harzstark AL, Ferrone M, van Criekinge M, Chang JW, Bok R, Park I, Reed G, Carvajal L, Small EJ, Munster P, Weinberg VK, Ardenkjaer-Larsen JH, Chen AP, Hurd RE, Odegardstuen LI, Robb FJ, Tropp J, Murray JA (August 2013). "Metabolic imaging of patients with prostate cancer using hyperpolarized [1-¹³C]pyruvate". Science Translational Medicine. 5 (198): 198ra108. doi:10.1126/scitranslmed.3006070. PMC 4201045. PMID 23946197.
- Jóhannesson H, Macholl S, Ardenkjaer-Larsen JH (April 2009). "Dynamic Nuclear Polarization of [1-13C]pyruvic acid at 4.6 tesla". Journal of Magnetic Resonance. 197 (2): 167–75. Bibcode:2009JMagR.197..167J. doi:10.1016/j.jmr.2008.12.016. PMID 19162518.
- Miloushev VZ, Keshari KR, Holodny AI (February 2016). "Hyperpolarization MRI: Preclinical Models and Potential Applications in Neuroradiology". Topics in Magnetic Resonance Imaging. 25 (1): 31–7. doi:10.1097/RMR.0000000000000076. PMC 4968075. PMID 26848559.
- "Clinical Trials".
- Lupo JM, Chen AP, Zierhut ML, Bok RA, Cunningham CH, Kurhanewicz J, Vigneron DB, Nelson SJ (February 2010). "Analysis of hyperpolarized dynamic 13C lactate imaging in a transgenic mouse model of prostate cancer". Magnetic Resonance Imaging. 28 (2): 153–62. doi:10.1016/j.mri.2009.07.007. PMC 3075841. PMID 19695815.
- Cunningham CH, Chen AP, Lustig M, Hargreaves BA, Lupo J, Xu D, Kurhanewicz J, Hurd RE, Pauly JM, Nelson SJ, Vigneron DB (July 2008). "Pulse sequence for dynamic volumetric imaging of hyperpolarized metabolic products". Journal of Magnetic Resonance. 193 (1): 139–46. Bibcode:2008JMagR.193..139C. doi:10.1016/j.jmr.2008.03.012. PMC 3051833. PMID 18424203.
- Larson PE, Kerr AB, Chen AP, Lustig MS, Zierhut ML, Hu S, Cunningham CH, Pauly JM, Kurhanewicz J, Vigneron DB (September 2008). "Multiband excitation pulses for hyperpolarized 13C dynamic chemical-shift imaging". Journal of Magnetic Resonance. 194 (1): 121–7. Bibcode:2008JMagR.194..121L. doi:10.1016/j.jmr.2008.06.010. PMC 3739981. PMID 18619875.
- Marco-Rius I, Cao P, von Morze C, Merritt M, Moreno KX, Chang GY, Ohliger MA, Pearce D, Kurhanewicz J, Larson PE, Vigneron DB (April 2017). "13 C-MR metabolism studies". Magnetic Resonance in Medicine. 77 (4): 1419–1428. doi:10.1002/mrm.26226. PMC 5040611. PMID 27017966.
- Xing Y, Reed GD, Pauly JM, Kerr AB, Larson PE (September 2013). "Optimal variable flip angle schemes for dynamic acquisition of exchanging hyperpolarized substrates". Journal of Magnetic Resonance. 234: 75–81. Bibcode:2013JMagR.234...75X. doi:10.1016/j.jmr.2013.06.003. PMC 3765634. PMID 23845910.
- Maidens J, Gordon JW, Arcak M, Larson PE (November 2016). "Optimizing Flip Angles for Metabolic Rate Estimation in Hyperpolarized Carbon-13 MRI". IEEE Transactions on Medical Imaging. 35 (11): 2403–2412. doi:10.1109/TMI.2016.2574240. PMC 5134417. PMID 27249825.
- Chattergoon N, Martínez-Santiesteban F, Handler WB, Ardenkjaer-Larsen JH, Scholl TJ (January 2013). "Field dependence of T1 for hyperpolarized [1-13C]pyruvate". Contrast Media & Molecular Imaging. 8 (1): 57–62. doi:10.1002/cmmi.1494. PMID 23109393.
- Yen YF, Le Roux P, Mayer D, King R, Spielman D, Tropp J, Butts Pauly K, Pfefferbaum A, Vasanawala S, Hurd R (May 2010). "T(2) relaxation times of (13)C metabolites in a rat hepatocellular carcinoma model measured in vivo using (13)C-MRS of hyperpolarized [1-(13)C]pyruvate". NMR in Biomedicine. 23 (4): 414–23. doi:10.1002/nbm.1481. PMC 2891253. PMID 20175135.
- Søgaard LV, Schilling F, Janich MA, Menzel MI, Ardenkjaer-Larsen JH (May 2014). "In vivo measurement of apparent diffusion coefficients of hyperpolarized ¹³C-labeled metabolites". NMR in Biomedicine. 27 (5): 561–9. doi:10.1002/nbm.3093. PMID 24664927.
- Bahrami N, Swisher CL, Von Morze C, Vigneron DB, Larson PE (February 2014). "Kinetic and perfusion modeling of hyperpolarized (13)C pyruvate and urea in cancer with arbitrary RF flip angles". Quantitative Imaging in Medicine and Surgery. 4 (1): 24–32. doi:10.3978/j.issn.2223-4292.2014.02.02. PMC 3947982. PMID 24649432.
- Hill DK, Orton MR, Mariotti E, Boult JK, Panek R, Jafar M, Parkes HG, Jamin Y, Miniotis MF, Al-Saffar NM, Beloueche-Babari M, Robinson SP, Leach MO, Chung YL, Eykyn TR (2014). "Model free approach to kinetic analysis of real-time hyperpolarized 13C magnetic resonance spectroscopy data". PLOS One. 8 (9): e71996. Bibcode:2013PLoSO...871996H. doi:10.1371/journal.pone.0071996. PMC 3762840. PMID 24023724.