Propylene oxide
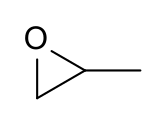
Propylene oxide is an organic compound with the molecular formula CH3CHCH2O. This colourless volatile liquid with an odour resembling ether, is produced on a large scale industrially. Its major application is its use for the production of polyether polyols for use in making polyurethane plastics. It is a chiral epoxide, although it is commonly used as a racemic mixture.
 | |
-Propylene_oxide_molecule_ball.png) | |
| Names | |
|---|---|
| Preferred IUPAC name
2-Methyloxirane | |
| Other names
Propylene oxide Epoxypropane Propylene epoxide 1,2-Propylene oxide Methyl oxirane 1,2-Epoxypropane Propene oxide Methyl ethylene oxide Methylethylene oxide | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.000.800 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C3H6O | |
| Molar mass | 58.080 g·mol−1 |
| Appearance | Colorless liquid |
| Odor | benzene-like[1] |
| Density | 0.859 g/cm3[2] |
| Melting point | −111.9 °C (−169.4 °F; 161.2 K)[2] |
| Boiling point | 35 °C (95 °F; 308 K)[2] |
| 41% (20 °C)[1] | |
| Vapor pressure | 445 mmHg (20 °C)[1] |
| −4.25×10−5 cm3/mol[3] | |
Refractive index (nD) |
1.3660[2] |
| Thermochemistry | |
Heat capacity (C) |
120.4 J·(K·mol)−1 |
Std molar entropy (S |
196.5 J·(K·mol)−1 |
Std enthalpy of formation (ΔfH⦵298) |
−123.0 kJ·mol−1[4] |
| Hazards | |
| Main hazards | Extremely flammable[5][6] |
| GHS pictograms |    |
| GHS Signal word | Danger |
| NFPA 704 (fire diamond) | |
| Flash point | −37 °C (−35 °F; 236 K) |
| 747 °C (1,377 °F; 1,020 K) | |
| Explosive limits | 2.3–36%[1] |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
660 mg/kg (guinea pig, oral) 380 mg/kg (rat, oral) 440 mg/kg (mouse, oral) 1140 mg/kg (rat, oral) 690 mg/kg (guinea pig, oral)[7] |
LC50 (median concentration) |
1740 ppm (mouse, 4 h) 4000 ppm (rat, 4 h)[7] |
LCLo (lowest published) |
2005 ppm (dog, 4 h) 4000 ppm (guinea pig, 4 h)[7] |
| NIOSH (US health exposure limits): | |
PEL (Permissible) |
TWA 100 ppm (240 mg/m3)[1] |
REL (Recommended) |
Ca[1] |
IDLH (Immediate danger) |
Ca [400 ppm][1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
This compound is sometimes called 1,2-propylene oxide to distinguish it from its isomer 1,3-propylene oxide, better known as oxetane.
Production
Industrial production of propylene oxide starts from propylene.[8]Two general approaches are employed, one involving hydrochlorination and the other involving oxidation.[9] In 2005, about half of the world production was through chlorohydrin technology and one half via oxidation routes. The latter approach is growing in importance.[10]
Hydrochlorination route
The traditional route proceeds via the conversion of propene to propylene chlorohydrin according to the following simplified scheme:
The mixture of 1-chloro-2-propanol and 2-chloro-1-propanol is then dehydrochlorinated. For example:
Lime (calcium hydroxide) is often used to absorb the HCl.
Oxidation of propylene
The other general route to propylene oxide involves oxidation of propylene with an organic peroxide. The reaction follows this stoichiometry:
- CH3CH=CH2 + RO2H → CH3CHCH2O + ROH
The process is practiced with four hydroperoxides:[10]
- t-Butyl hydroperoxide derived from oxygenation of isobutane, which affords t-butanol. This coproduct can be dehydrated to isobutene, converted to MTBE, an additive for gasoline.
- Ethylbenzene hydroperoxide, derived from oxygenation of ethylbenzene, which affords 1-phenylethanol. This coproduct can be dehydrated to give styrene, a useful monomer.
- Cumene hydroperoxide derived from oxygenation of cumene (isopropylbenzene), which affords cumyl alcohol. Via dehydration and hydrogenation this coproduct can be recycled back to cumene. This technology was commercialized by Sumitomo Chemical.[11]
- Hydrogen peroxide is the oxidant in the hydrogen peroxide to propylene oxide (HPPO) process, catalyzed by a titanium-doped silicalite:
- C3H6 + H2O2 → C3H6O + H2O
In principle, this process produces only water was a side product. In practice, some ring-opened derivatives of PO are generated.[12]
Reactions
Like other epoxides, PO undergoes ring-opening reactions. With water, propylene glycol is produced. With alcohols, reactions, called hydroxylpropylation, analogous to ethoxylation occur. Grignard reagents add to propylene oxide to give secondary alcohols.
Some other reactions of propylene oxide include:[13]
- Reaction with aluminium oxide at 250–260 °C leads to propionaldehyde and a little acetone.
- Reaction with silver(I) oxide leads to acetic acid.
- Reaction with sodium–mercury amalgam and water leads to isopropanol.
Uses
Between 60 and 70% of all propylene oxide is converted to polyether polyols by the process called alkoxylation.[14] These polyols are building blocks in the production of polyurethane plastics.[15] About 20% of propylene oxide is hydrolyzed into propylene glycol, via a process which is accelerated by acid or base catalysis. Other major products are polypropylene glycol, propylene glycol ethers, and propylene carbonate.
Niche uses
Fumigant
The United States Food and Drug Administration has approved the use of propylene oxide to pasteurize raw almonds beginning on September 1, 2007, in response to two incidents of contamination by Salmonella in commercial orchards, one incident occurring in Canada and one in the United States.[16][17] Pistachio nuts can also be subjected to propylene oxide to control Salmonella.
Microscopy
Propylene oxide is commonly used in the preparation of biological samples for electron microscopy, to remove residual ethanol previously used for dehydration. In a typical procedure, the sample is first immersed in a mixture of equal volumes of ethanol and propylene oxide for 5 minutes, and then four times in pure oxide, 10 minutes each.
Safety
It is a probable human carcinogen, and is included into the List of IARC Group 2B carcinogens.[18]
Natural occurrence
In 2016 it was reported that propylene oxide was detected in Sagittarius B2, a cloud of gas in the Milky Way weighing three million solar masses. It is the first chiral molecule to be detected in space.[19]
References
- NIOSH Pocket Guide to Chemical Hazards. "#0538". National Institute for Occupational Safety and Health (NIOSH).
- Haynes 2011, p. 3.384
- Haynes 2011, p. 3.577
- Haynes 2011, p. 5.24
- "NFPA DIAMOND". www.otrain.com.
- GOV, NOAA Office of Response and Restoration, US. "PROPYLENE OXIDE | CAMEO Chemicals | NOAA". cameochemicals.noaa.gov.
- "Propylene oxide". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- Nijhuis, T. Alexander; Makkee, Michiel; Moulijn, Jacob A.; Weckhuysen, Bert M. (2006). "The Production of Propene Oxide: Catalytic Processes and Recent Developments". Industrial & Engineering Chemistry Research. 45 (10): 3447–3459. doi:10.1021/ie0513090.
- Kahlich, Dietmar; Wiechern, Uwe; Lindner, Jörg. "Propylene Oxide". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a22_239.
- Nijhuis, T. Alexander; Makkee, Michiel; Moulijn, Jacob A.; Weckhuysen, Bert M. (2006). "The Production of Propene Oxide: Catalytic Processes and Recent Developments". Industrial & Engineering Chemistry Research. 45 (10): 3447. doi:10.1021/ie0513090. hdl:1874/20149.
- "Summary of Sumitomo process from Nexant Reports". Archived from the original on 2006-01-17. Retrieved 2007-09-18.
- Russo, V.; Tesser, R.; Santacesaria, E.; Di Serio, M. (2013). "Chemical and Technical Aspects of Propene Oxide Production via Hydrogen Peroxide (HPPO Process)". Industrial & Engineering Chemistry Research. 52 (3): 1168–1178. doi:10.1021/ie3023862.
- Heilbron, Ian, ed. (1953). Dictionary of Organic Compounds. 4. Oxford University Press. p. 249.
- Adam, Norbert; et al. "Polyurethanes". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a21_665.pub2.
- "Usage of proplyene oxide". Dow Chemical. Archived from the original on 2007-09-15. Retrieved 2007-09-10.
- "Guidance for Industry: Measures to Address the Risk for Contamination by Salmonella Species in Food Containing a Pistachio-Derived Product As An Ingredient; Draft Guidance". fda.gov. June 2009. Archived from the original on 2011-02-09.
- Agricultural Marketing Service, USDA (30 March 2007). "Almonds Grown in California; Outgoing Quality Control Requirements" (PDF). Federal Register. 72 (61): 15, 021–15, 036. Archived from the original (PDF) on 28 September 2007. Retrieved 2007-08-22.
- Grana, R.; Benowitz, N.; Glantz, S. A. (13 May 2014). "E-cigarettes: a scientific review". Circulation. 129 (19): 1972–1986. doi:10.1161/circulationaha.114.007667. PMC 4018182. PMID 24821826.
- "Scientists just detected this life-forming molecule in interstellar space for the first time". Science Alert. 2016-06-15.
Cited sources
- Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL: CRC Press. ISBN 1439855110.
External links
- WebBook page for C3H6O
- Propylene oxide at the United States Environmental Protection Agency
- Propylene oxide – chemical product info: properties, production, applications.
- Propylene oxide at the Technology Transfer Network Air Toxics Web Site
- CDC – NIOSH Pocket Guide to Chemical Hazards
